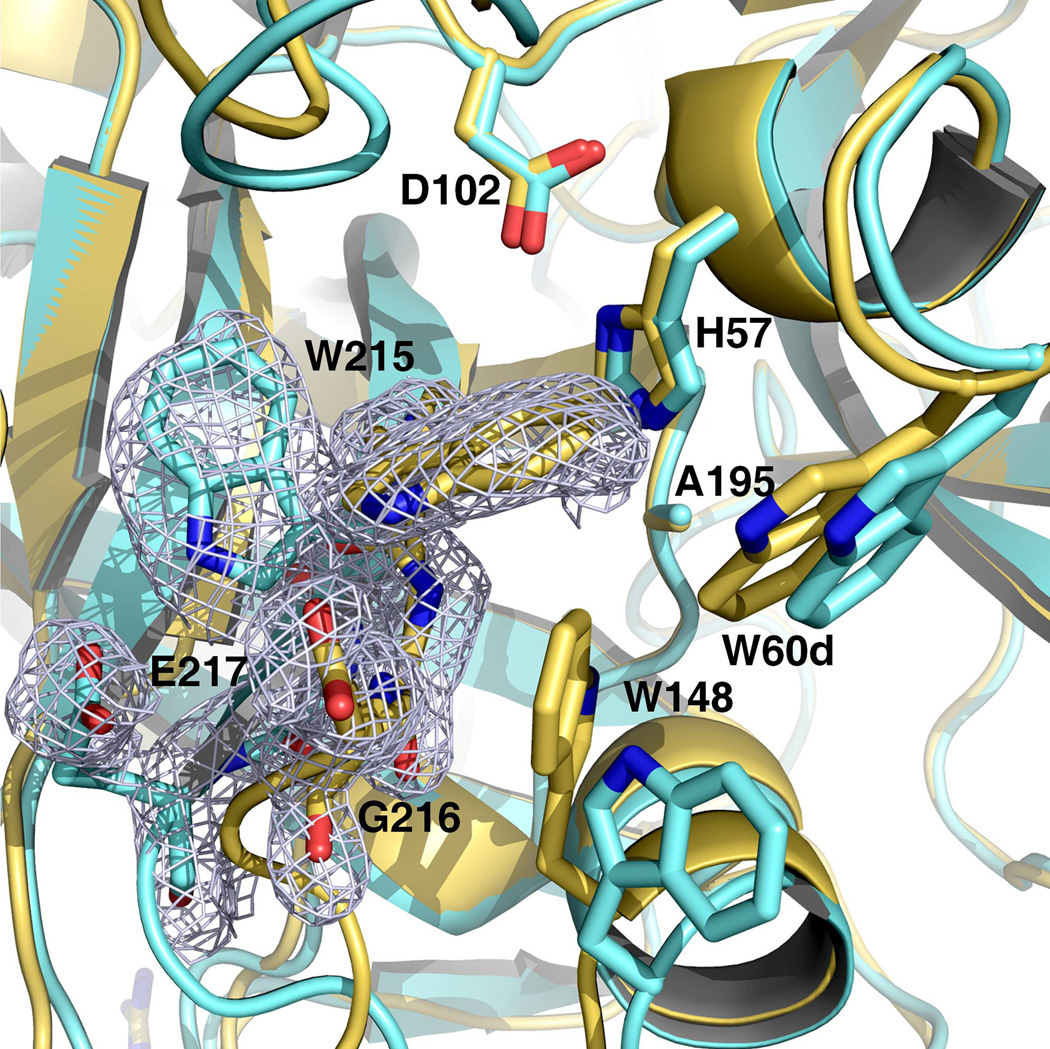
Figure 2. Active site accessibility in prethrombin-2.
The collapsed form of prethrombin-2 (orange) features a cluster of hydrophobic/aromatic residues that completely occludes access to the active site, as observed in the recent structure of prethrombin-1 (17). The cluster is formed by the collapse of W215 and W148 into the active site against W60d, with the indole ring of W215 moving >10 Å relative to its position in the open form (yellow). The electron density 2F0-Fc map (green mesh) outlines the alternative positions of W215 and is contoured at 1 σ.