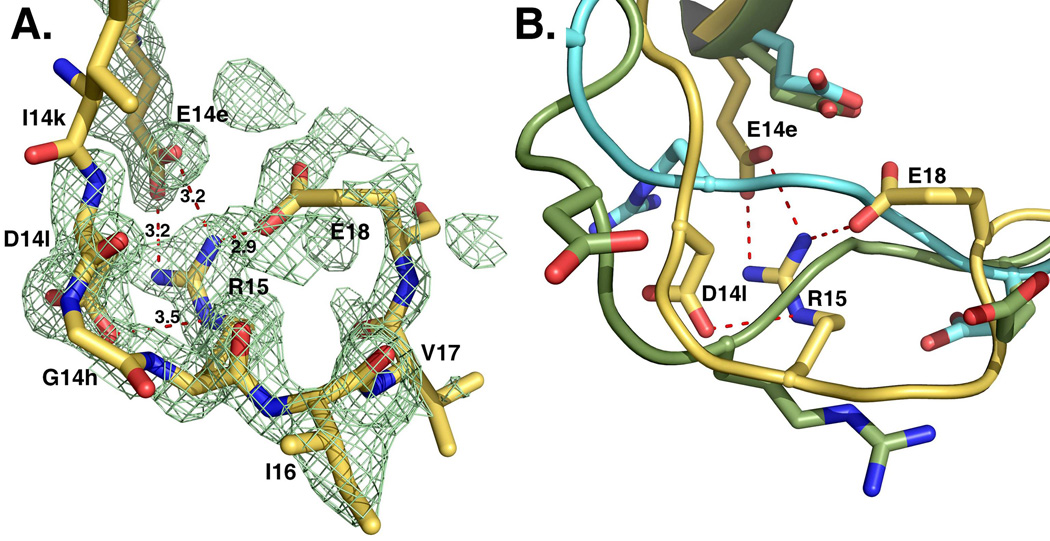
Figure 3. Activation domain of prethrombin-2.
The segment around the cleavage site at R15 defines the activation domain and shows an intact R15-I16 peptide bond. R15 is buried inside the protein in electrostatic interaction with the side chains of E14e, D14l and E18. These interactions are shown for the open conformation, but are equivalent to those observed in the collapsed form. The orientation of R15 in the free form of prethrombin-2 is unprecedented among existing crystal structures of zymogens of trypsin-like proteases, including structures of prethrombin-2 bound to hirugen, PPACK or staphylocoagulase (9, 18). The electron density 2F0-Fc map (green mesh) is contoured at 1 σ.