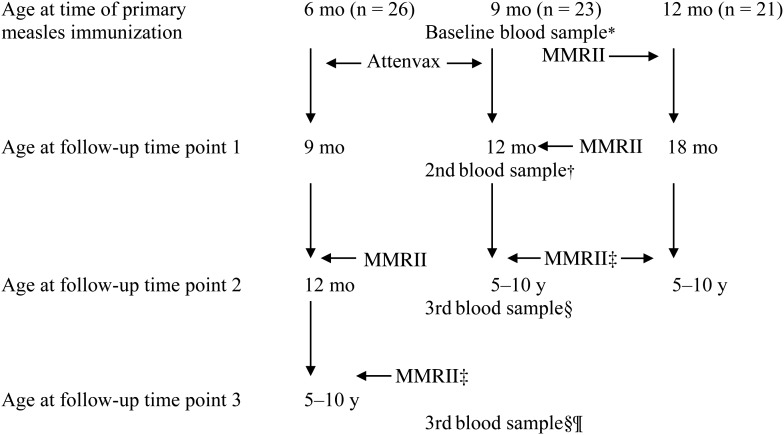
Figure 1.
Study design representing the timing of initial and subsequent measles vaccine doses and immune evaluations for each cohort. *Blood samples were collected on all infants, as previously reported [13], and used to determine passive antibody concentrations prior to primary measles immunization. †Blood samples were collected on all infants, as previously reported [13]. ‡Measles, mumps, and rubella virus vaccine live (MMRII) given at 5 years. §Blood samples were collected at random time points from children initially immunized at 9 (n = 23) or 12 (n = 21) months of age and who were now aged 5–10 years. ¶Blood samples were collected at random time points from children initially immunized at 6 months of age (n = 26) and who were now aged 5–10 years. Abbreviation: MMRII, measles, mumps, and rubella virus vaccine live.