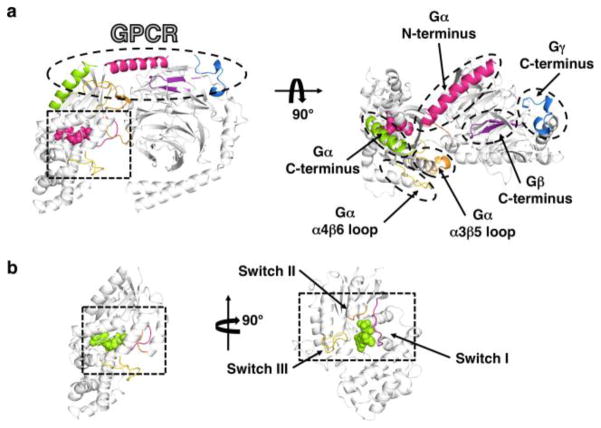
Figure 3. The binding regions of G proteins.
Heterotrimeric G proteins are composed of 3 subunits: the nucleotide-binding Gα and the dimeric Gβγ subunits. a) In the heterotrimer (PDBID 1GOT; (5)), the receptor-binding surface (oval) is composed of the N-terminus, C-terminus, α4β6 loop, α3β5 loop of the Gα subunit and the C-termini of the Gβ and Gγ subunits. The switch regions on the Gα subunit (box) are in conformations that do not bind to effectors. b) Interactions with activated receptors catalyzes nucleotide exchange in the guanine nucleotide binding site of the Gα subunit (PDBID 1TAD; (16)), and the heterotrimer disassociates into GTP-bound Gα and Gβγ The binding of GTP changes the conformation of the switch regions (box) and the disassociation of the complex leaves this surface unobstructed, allowing Switch II and the surrounding region to interact with effectors.