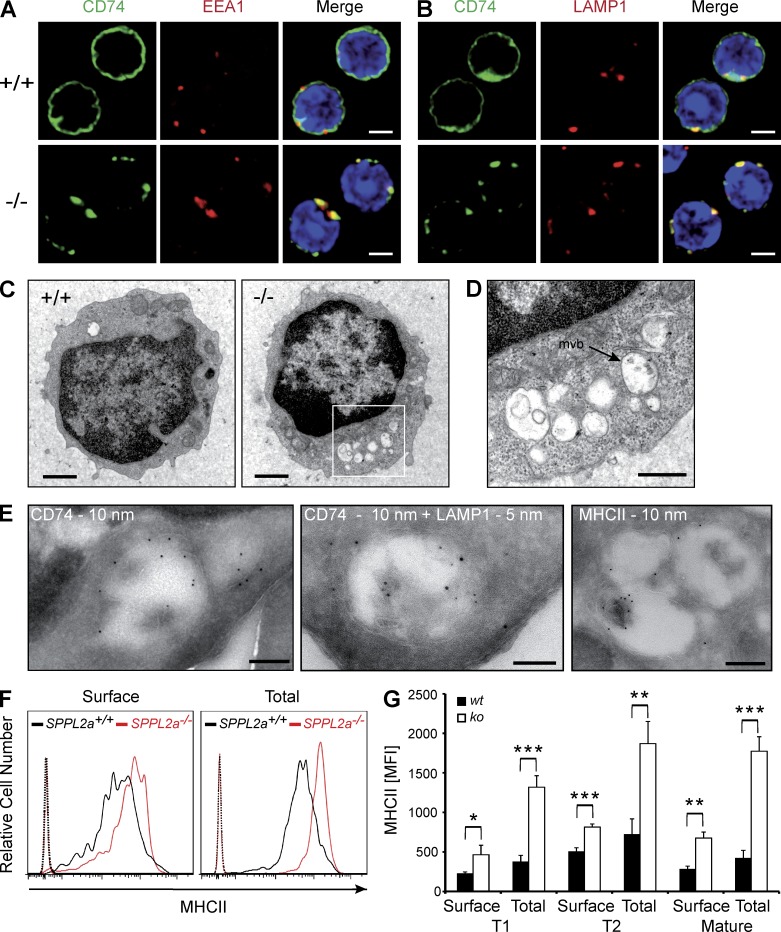
Figure 4.
Disturbance of membrane traffic within the endocytic system of SPPL2a−/− B cells. (A and B) Visualization of CD74 in isolated splenic SPPL2a+/+ and SPPL2a−/− B cells by indirect immunofluorescence using an antibody against an N-terminal epitope detecting the NTF and the full-length protein. EEA1 (A) and LAMP1 (B) served as markers of early endosomes and lysosomes/late endosomes, respectively. Bars, 2 µm. (C) Transmission electron microscopy of splenic IgM+ B cells from wild-type or SPPL2a−/− mice. Bars, 1 µm. (D) Vacuoles in SPPL2a−/− B cells exhibited various contents of low-electron density. Occasionally, multivesicular bodies (mvb) were observed (arrow). Bar, 500 nm. (E) Presence of CD74 NTF, LAMP1, and MHCII in vacuoles of IgM+ B cells from SPPL2a−/− mice was assessed by immunogold labeling. Bars, 200 nm (CD74 and MHCII single labeling) or 100 nm (CD74 + LAMP1 double labeling). (F and G) Surface and total MHCII levels in transitional stage T1 B cells (B220+ CD21low CD24high) of SPPL2a-deficient or wild-type mice. Splenocytes were stained for B220, CD21, and CD24, allowing for identification of B cell subsets. Subsequently, cells were incubated with anti-MHCII with or without previous permeabilization and analyzed by flow cytometry. Surface and total MHCII levels are shown as histograms representative of three independent experiments or as mean of median fluorescence intensity (MFI) from three mice per genotype (G). ***, P < 0.001; **, P < 0.01; *, P < 0.05, unpaired, two-tailed Student’s t test.