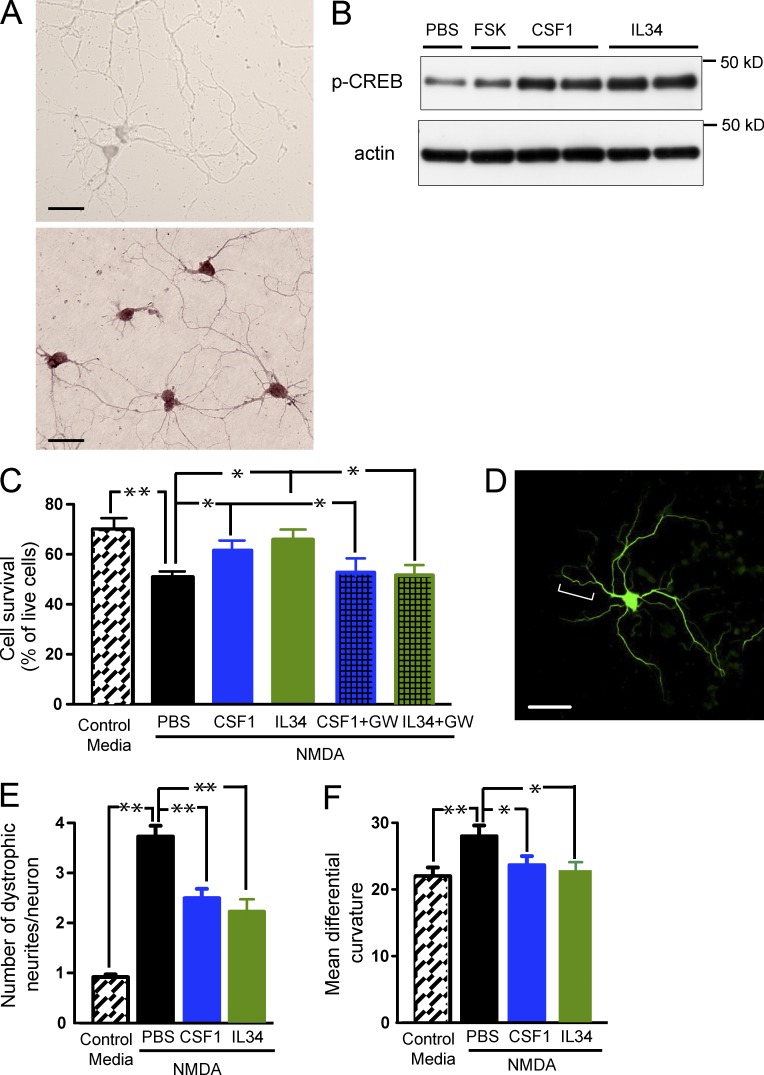
Figure 9.
Recombinant CSF1 and IL-34 inhibit excitotoxic injury and activate the CREB pathway in vitro. (A) Detection of Csf1r mRNA by in situ hybridization in cultured primary neurons. Primary hippocampal neurons were isolated from 16-d-old CF1 mouse embryos, aged for 6–7 d and hybridized with DIG-labeled Csf1r sense (top) or antisense (bottom) probes. (B–F) Primary hippocampal neurons were isolated from 16-d-old CF1 mouse embryos and were aged for 6–7 d (B and C) or 21–22 d (D–F). (B) Primary neurons were exposed to 1 or 100 ng/ml IL-34, 1 or 100 ng/ml CSF1, 10 µM Forskolin (FSK, as a positive control), or PBS (as a negative control) for 30 min. The cells were lysed and subjected to Western blot analysis for p-CREB. (C–F) Primary neurons were challenged with 100 µM NMDA in the presence and absence of CSF1 or IL-34 (both at 10 ng/ml) and assayed for neurotoxicity (C) or neuritic dystrophy (D–F), respectively. For inhibitor experiments, 10 µM GW2580 was coincubated with CSF1 or IL-34. (C) Live and dead cells were counted according to their morphologies determined by phase-contrast microscopy. Results are expressed as percentage of live cells. (D–F) Neurons were fixed and immunostained for MAP-2 to visualize dendrites. Dystrophic neurites in NMDA-treated cultures show increased tortuosity, exhibiting multiple abrupt turns (D, bracket). The number of dystrophic neurites (E) and their mean differential curvature (F) are reduced with CSF1 or IL-34 treatment. Error bars indicate SEM. Bars, 20 µm. *, P < 0.05; **, P < 0.01 compared by ANOVA and Tukey’s post-hoc test. The experiments were independently performed five times for B and twice for the rest with triplicates of each group.