Abstract
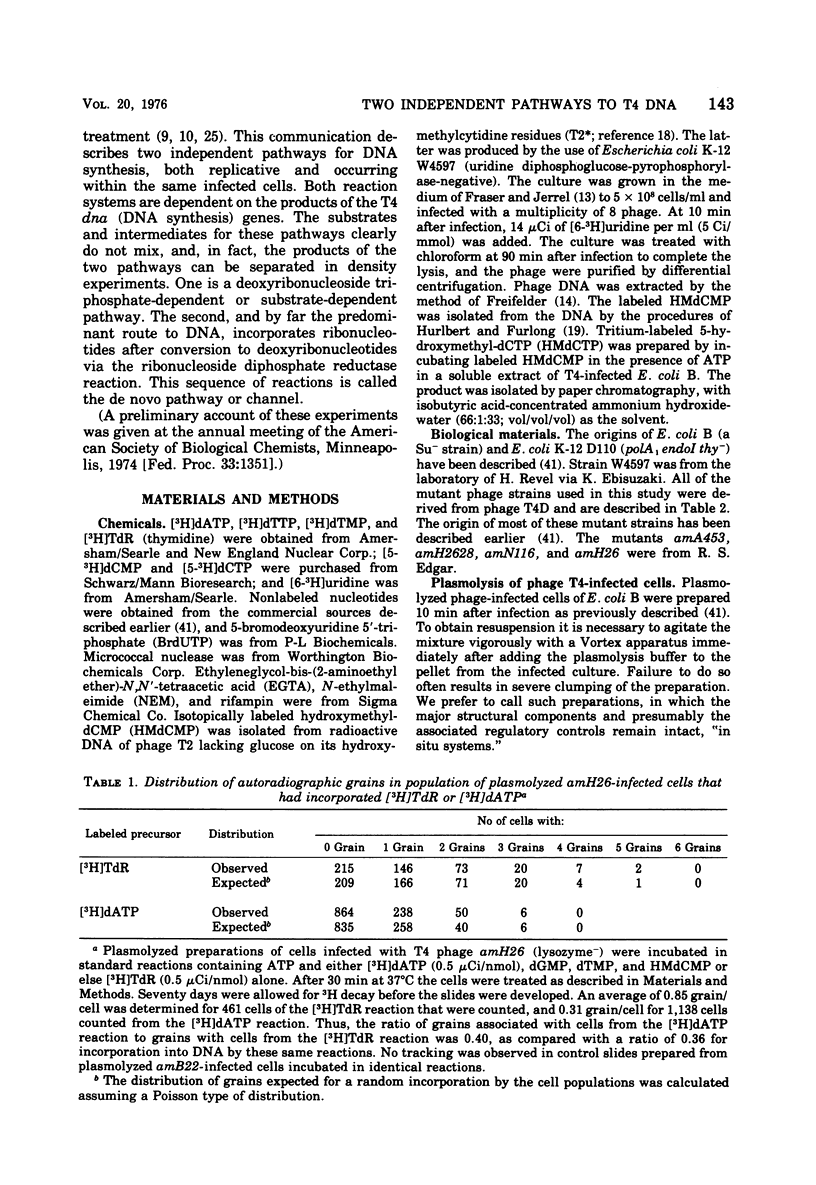
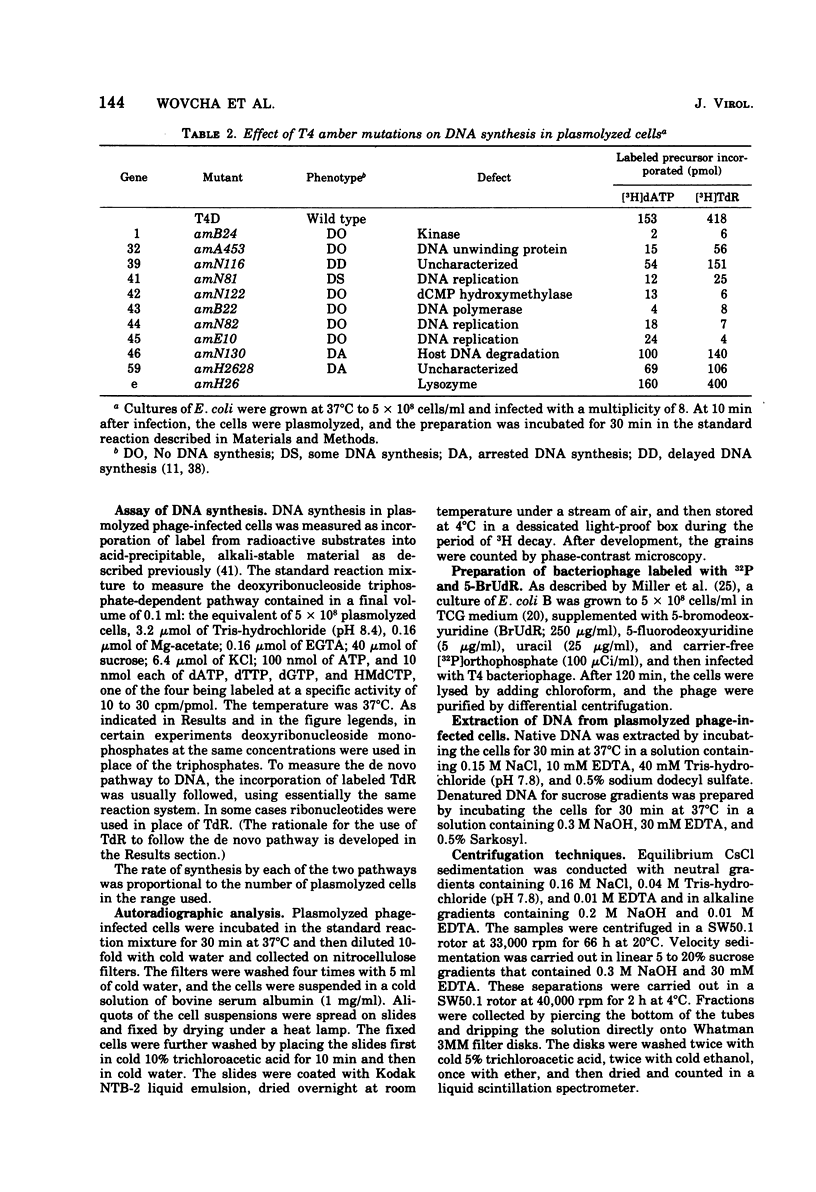
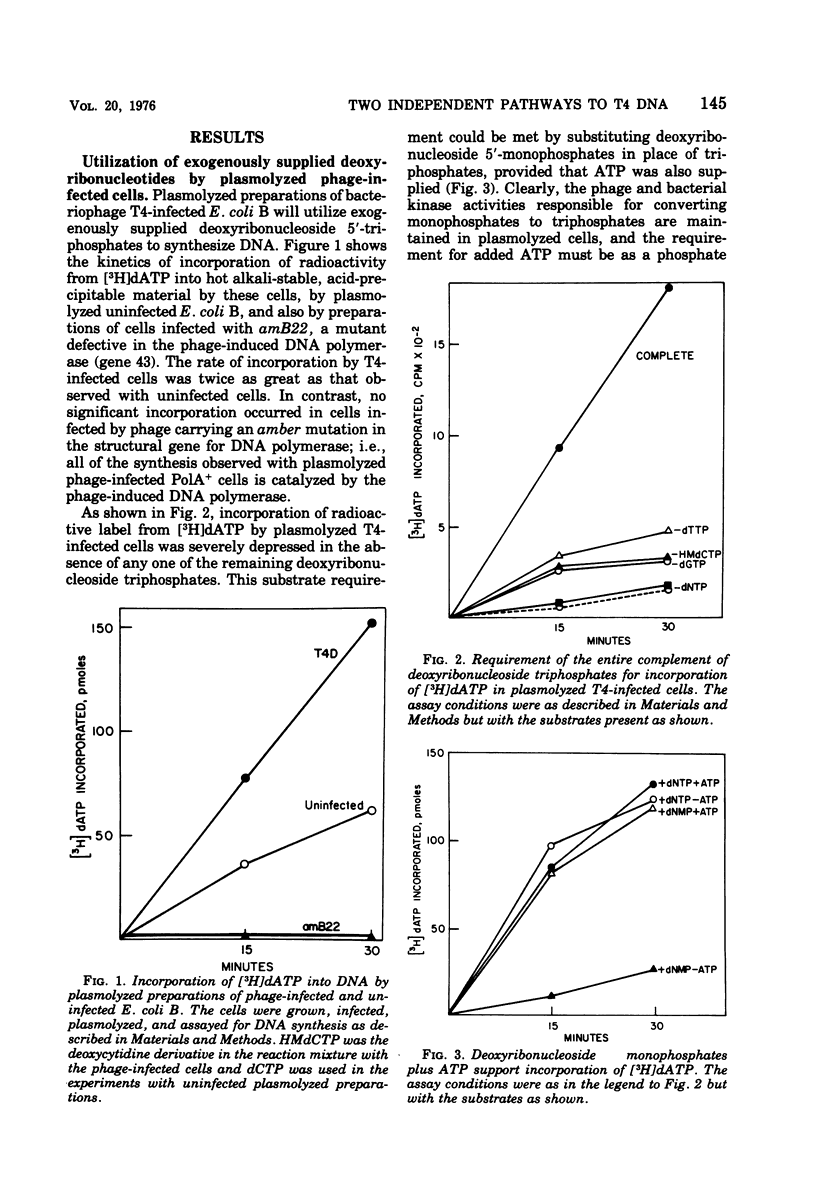
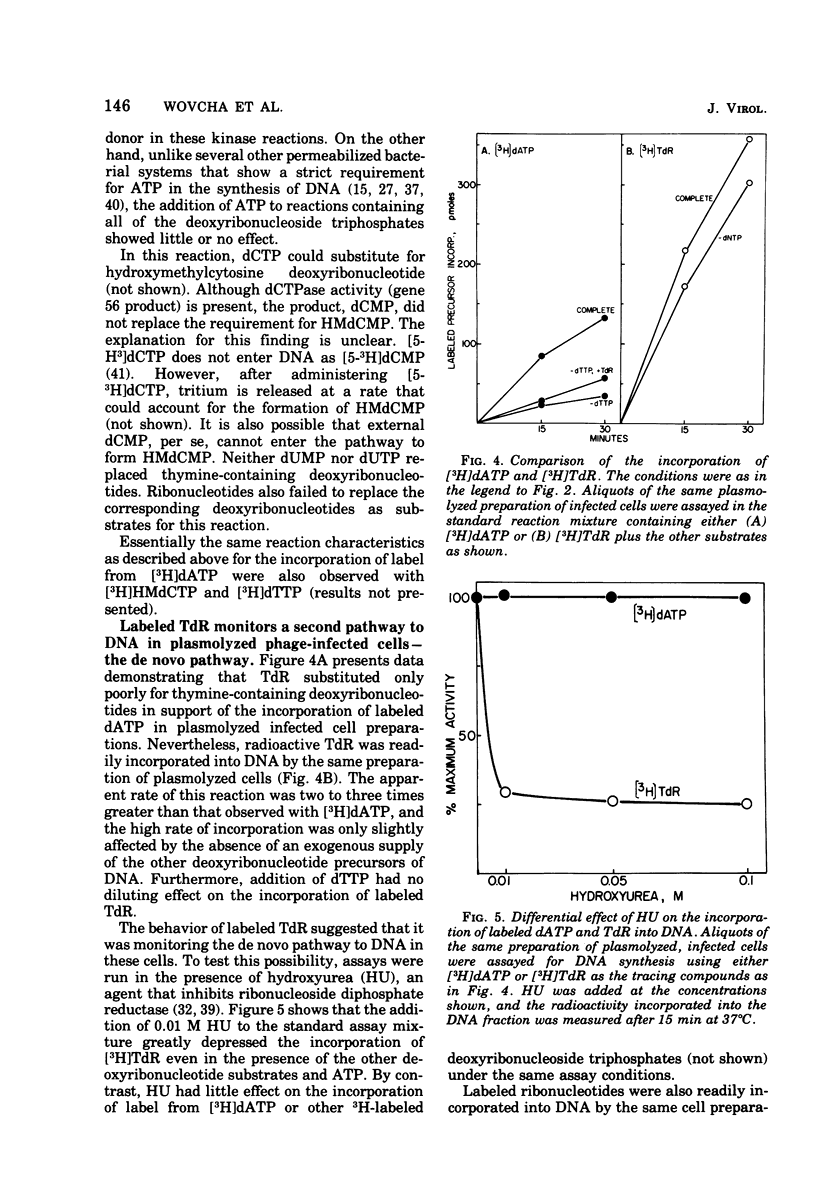
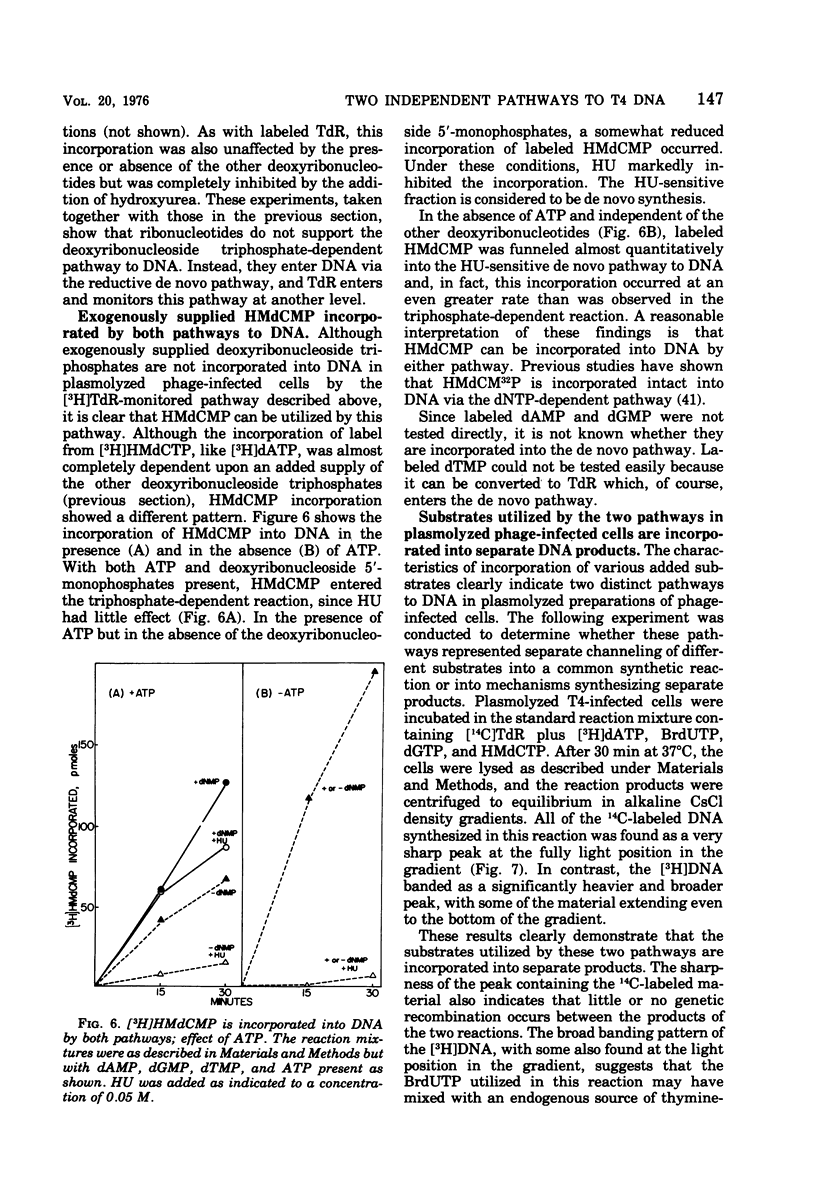
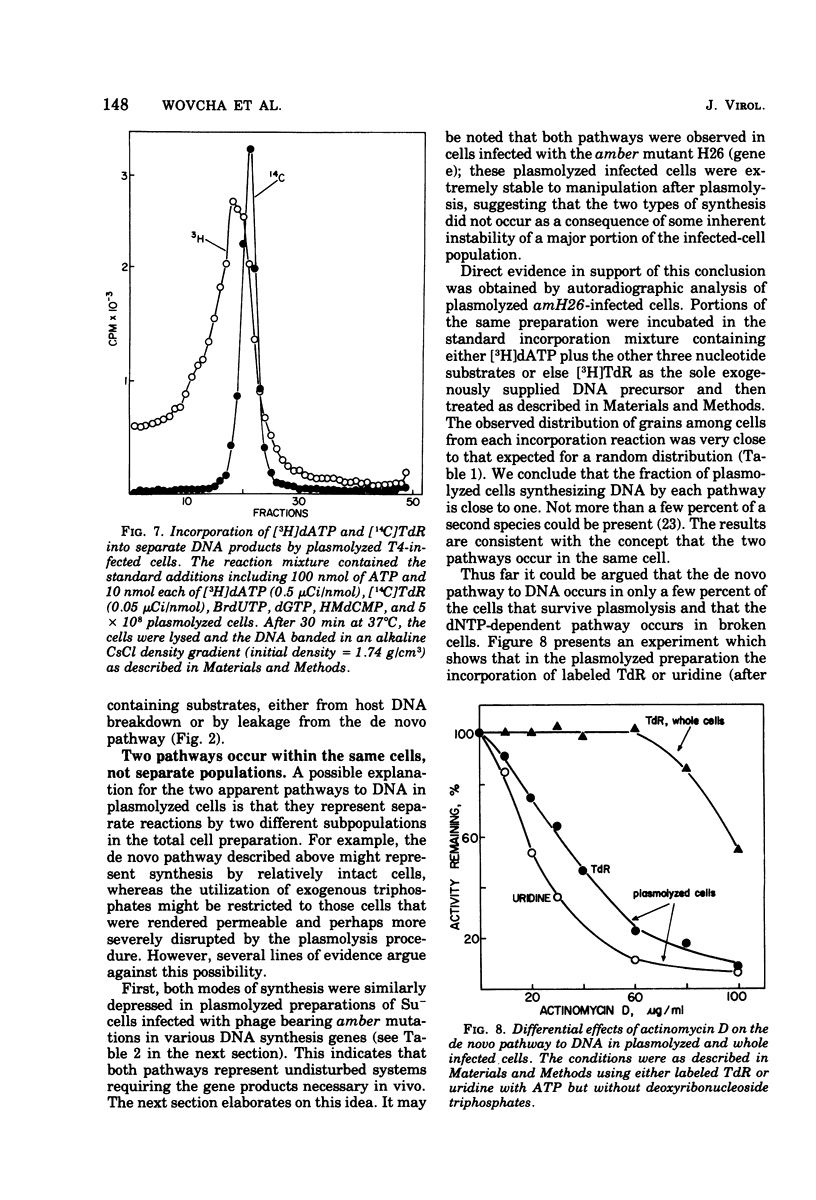
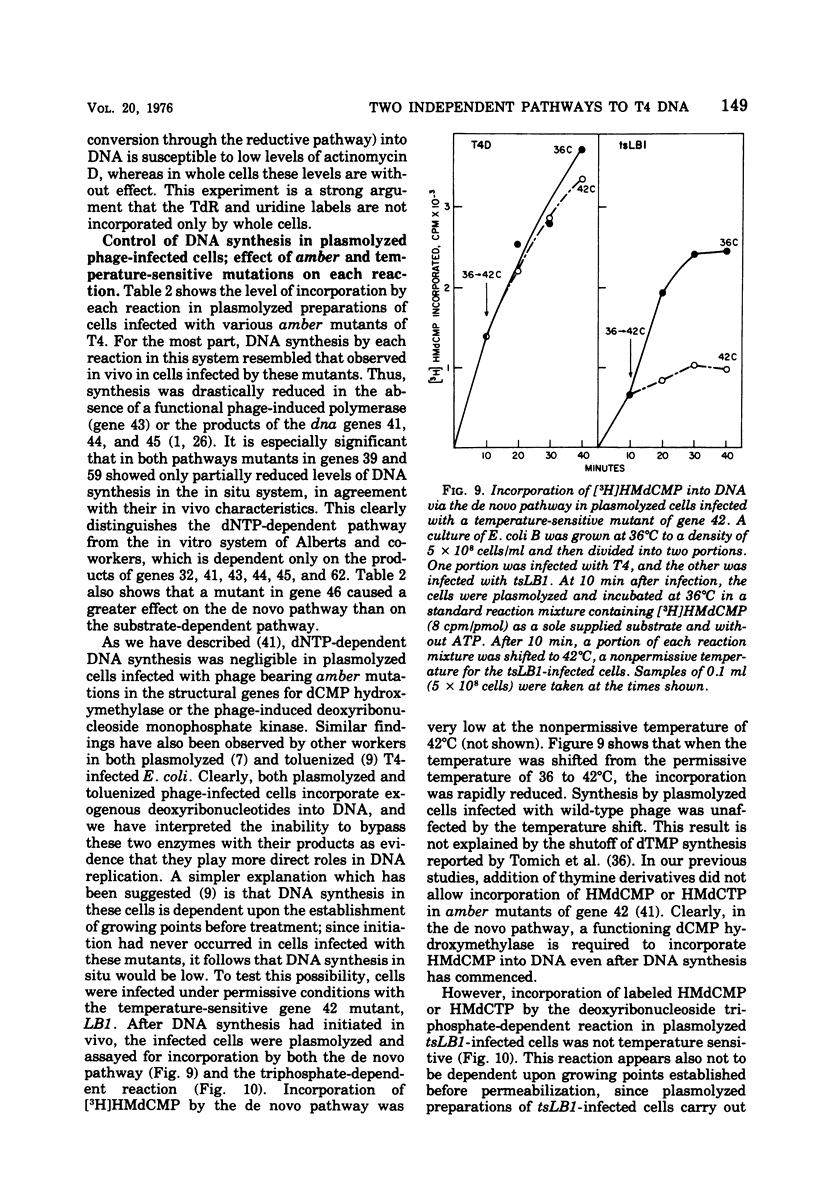
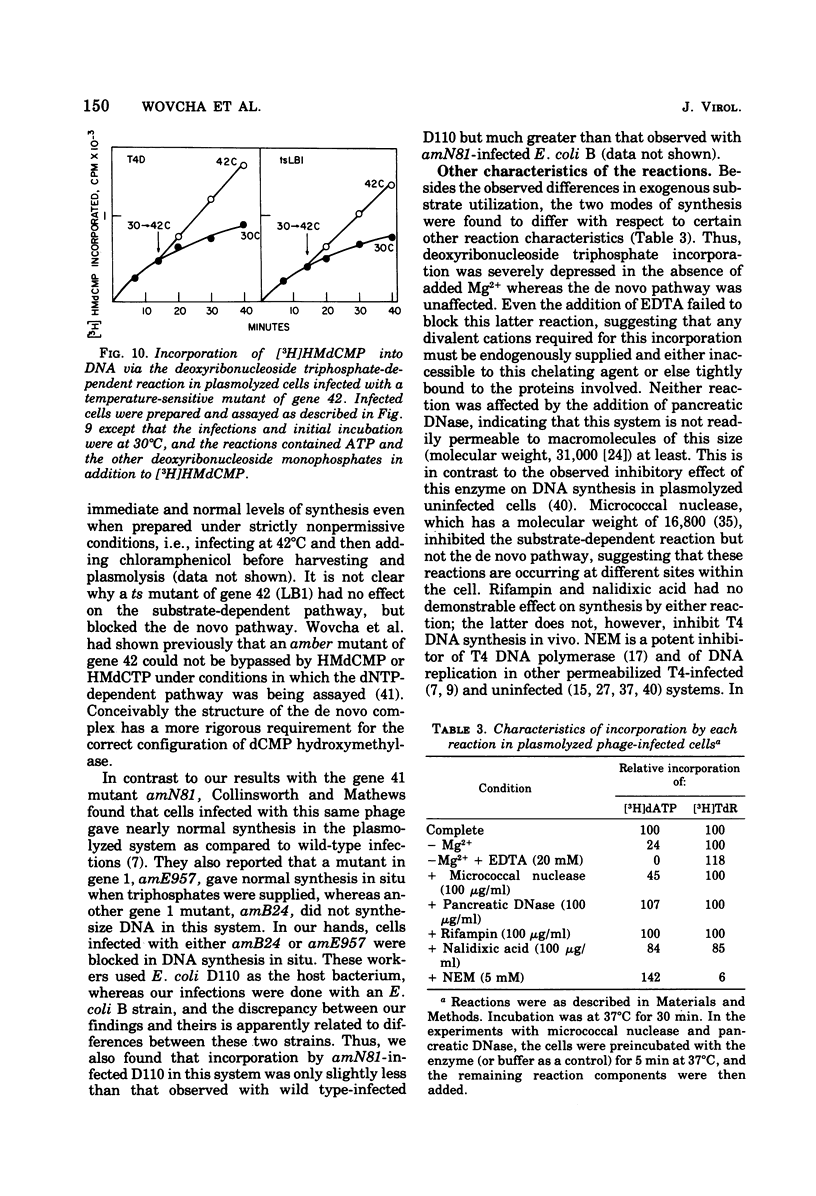
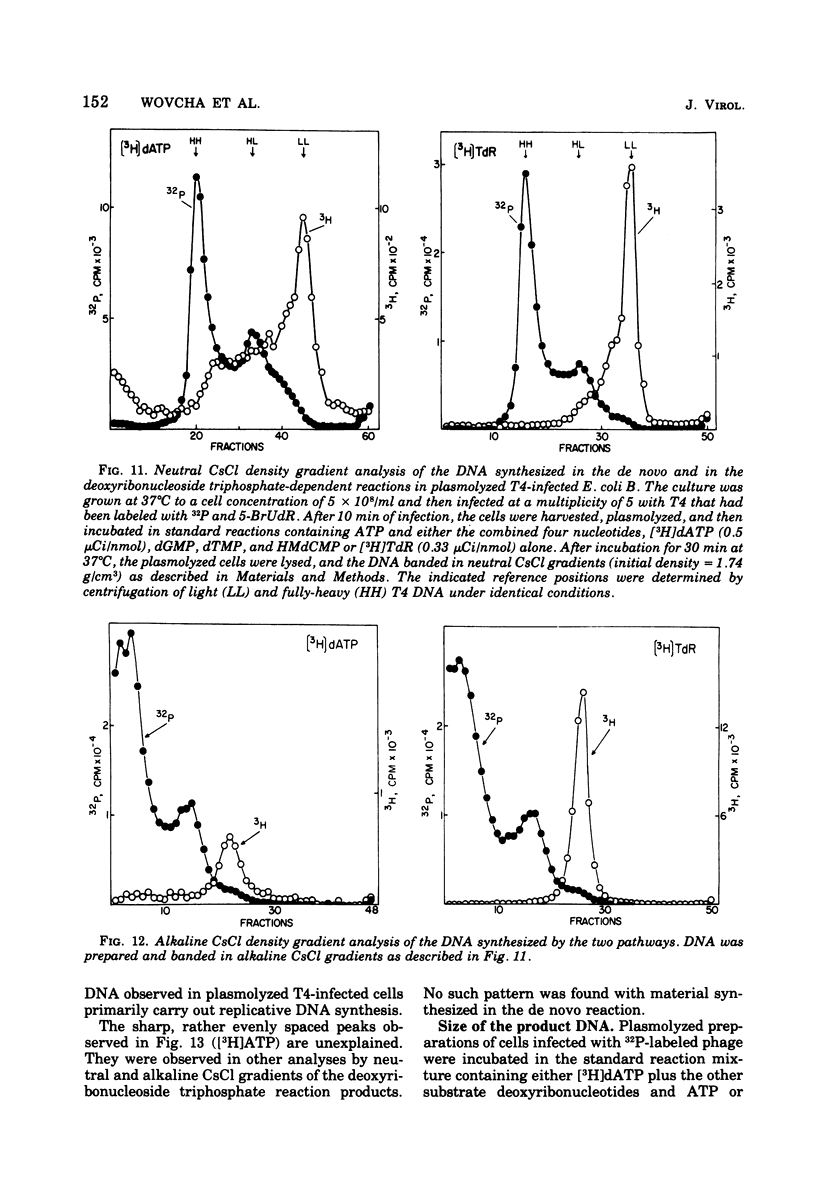
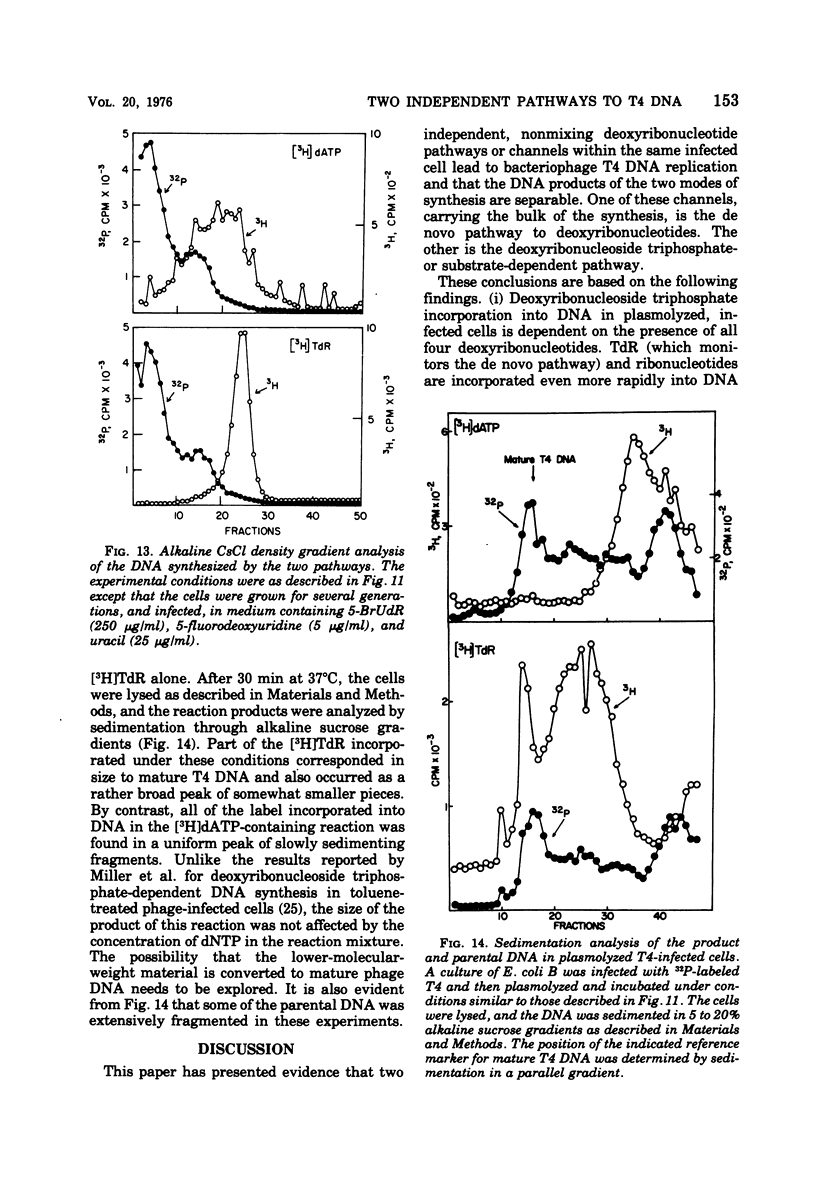
Bacteriophage T4-infected Escherichia coli rendered permeable to nucleotides by sucrose plasmolysis exhibited two apparently separate pathways or channels to T4 DNA with respect to the utilization of exogenously supplied substrates. By one pathway, individual labeled ribonucleotides, thymidine (tdR), and 5-hydroxymethyl-dCMP could be incorporated into phage DNA. Incorporation of each of these labeled compounds was not dependent upon the addition of the other deoxyribonucleotide precursors, suggesting that a functioning de novo pathway to deoxyribonucleotides was being monitored. The second pathway or reaction required all four deoxyribonucleoside triphosphates or the deoxyribonucleoside monophosphates together with ATP. However, in this reaction, dTTP was not replaced by TdR. The two pathways were also distinguished on the basis of their apparent Mg2+ requirements and responses to N-ethylmaleimide, micrococcal nuclease, and to hydroxyurea, which is a specific inhibitor of ribonucleoside diphosphate reductase. Separate products were synthesized by the two channels, as shown by density-gradient experiments and velocity sedimentation analysis. Each of the pathways required the products of the T4 DNA synthesis genes. Furthermore, DNA synthesis by each pathway appeared to be coupled to the functioning of several of the phage-induced enzymes involved in deoxyribonucleotide biosynthesis. Both systems represent replicative phage DNA synthesis as determined by CsCl density-gradient analysis. Autoradiographic and other studies provided evidence that both pathways occur in the same cell. Further studies were carried out on the direct role of dCMP hydroxymethylase in T4 DNA replication. Temperature-shift experiments in plasmolyzed cells using a temperature-sensitive mutant furnished strong evidence that this gene product is necessary in DNA replication and is not functioning by allowing preinitiation of DNA before plasmolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry J., Alberts B. In vitro complementation as an assay for new proteins required for bacteriophage T4 DNA replication: purification of the complex specified by T4 genes 44 and 62. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2717–2721. doi: 10.1073/pnas.69.9.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. II. Allosteric regulation of substrate sepecificity and catalytic activity. J Biol Chem. 1972 Nov 25;247(22):7276–7281. [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. III. Isolation and characterization of proteins B1 and B2. J Biol Chem. 1975 Sep 25;250(18):7450–7455. [PubMed] [Google Scholar]
- Billen D., Carreira L. B., Hadden C. T., Silverstein S. J. Evidence suggestive of compartmentalization of deoxyribonucleic acid-synthesizing systems in freeze-treated Bacillus subtilis. J Bacteriol. 1971 Dec;108(3):1250–1256. doi: 10.1128/jb.108.3.1250-1256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. S., Greenberg G. R. Evidence for a possible direct role of dCMP hydroxymethylase in T4 phage DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:351–359. doi: 10.1101/sqb.1968.033.01.041. [DOI] [PubMed] [Google Scholar]
- Chiu C. S., Tomich P. K., Greenberg G. R. Simultaneous initiation of synthesis of bacteriophage T4 DNA and of deoxyribonucleotides. Proc Natl Acad Sci U S A. 1976 Mar;73(3):757–761. doi: 10.1073/pnas.73.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinsworth W. L., Mathews C. K. Biochemistry of DNA-defective amber mutants of bacteriophage T4. IV. DNA synthesis in plasmolyzed cells. J Virol. 1974 Apr;13(4):908–915. doi: 10.1128/jvi.13.4.908-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dicou L., Cozzarelli N. R. Bacteriophage T4-directed DNA synthesis in toluene-treated cells. J Virol. 1973 Dec;12(6):1293–1302. doi: 10.1128/jvi.12.6.1293-1302.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Fleming W. H., Bessman M. J. The enzymology of virus-infected bacteria. IX. Purification and properties of the deoxycytidylate deaminase of T6-infected Escherichia coli. J Biol Chem. 1967 Feb 10;242(3):363–371. [PubMed] [Google Scholar]
- Ganesan A. T. Adenosine triphosphate-dependent synthesis of biologically active DNA by azide-poisoned bacteria. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1296–1300. doi: 10.1073/pnas.68.6.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goscin L. A., Hall D. H. Hydroxyurea-sensitive mutants of T4. II. Degradation and utilization of bacterial DNA. Virology. 1973 Nov;56(1):207–217. doi: 10.1016/0042-6822(73)90300-0. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- HATTMAN S., FUKASAWA T. HOST-INDUCED MODIFICATION OF T-EVEN PHAGES DUE TO DEFECTIVE GLUCOSYLATION OF THEIR DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:297–300. doi: 10.1073/pnas.50.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W. Fragmentary transfer of P32-labeled parental DNA to progeny phage. Virology. 1961 Jan;13:124–134. doi: 10.1016/0042-6822(61)90039-3. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., SZYBALSKI W. Dispersive transfer of the parental DNA molecule to the progeny of phage phiX-174. Virology. 1959 Oct;9:260–274. doi: 10.1016/0042-6822(59)90119-9. [DOI] [PubMed] [Google Scholar]
- Knippers R., Strätling W. The DNA replicating capacity of isolated E. coli cell wall-membrane complexes. Nature. 1970 May 23;226(5247):713–717. doi: 10.1038/226713a0. [DOI] [PubMed] [Google Scholar]
- Lindberg U. Molecular weight and amino acid composition of deoxyribonuclease I. Biochemistry. 1967 Jan;6(1):335–342. doi: 10.1021/bi00853a050. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr, Taylor D. M., MacKay K., Smith H. W. Replication of T4 DNA in Escherichia coli treated with toluene. J Virol. 1973 Dec;12(6):1195–1203. doi: 10.1128/jvi.12.6.1195-1203.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. F., Sinha N. K., Alberts B. M. Reconstruction of bacteriophage T4 DNA replication apparatus from purified components: rolling circle replication following de novo chain initiation on a single-stranded circular DNA template. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4800–4804. doi: 10.1073/pnas.72.12.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T. W., Stafford M. E., Mathews C. K. Biochemistry of DNA-defective mutants of bacteriophage T4. VI. Biological functions of gene 42. J Virol. 1976 Mar;17(3):973–982. doi: 10.1128/jvi.17.3.973-982.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Sugimoto K., Okazaki T., Imae Y., Sugino A. DNA chain growth: in vivo and in vitro synthesis in a DNA polymerase-negative mutant of E. coli. Nature. 1970 Oct 17;228(5268):223–226. doi: 10.1038/228223a0. [DOI] [PubMed] [Google Scholar]
- Schaller H., Otto B., Nüsslein V., Huf J., Herrmann R., Bonhoeffer F. Deoxyribonucleic acid replication in vitro. J Mol Biol. 1972 Jan 28;63(2):183–200. doi: 10.1016/0022-2836(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Scocca J. J., Panny S. R., Bessman M. J. Studies of deoxycytidylate deaminase from T4-infected Escherichia coli. J Biol Chem. 1969 Jul 10;244(13):3698–3706. [PubMed] [Google Scholar]
- Siegel P. J., Schaechter M. The role of the host cell membrane in the replication and morphogenesis of bacteriophages. Annu Rev Microbiol. 1973;27:261–282. doi: 10.1146/annurev.mi.27.100173.001401. [DOI] [PubMed] [Google Scholar]
- Sinha N. K., Snustad D. P. Mechanism of inhibition of deoxyribonucleic acid synthesis in Escherichia coli by hydroxyurea. J Bacteriol. 1972 Dec;112(3):1321–1324. doi: 10.1128/jb.112.3.1321-1334.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Schaller H. E., Bonhoeffer F. J. DNA synthesis in vitro. Nature. 1970 May 23;226(5247):711–713. doi: 10.1038/226711a0. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M13 DNA in plasmolysed Escherichia coli cells. Biochem Biophys Res Commun. 1973 Sep 18;54(2):578–584. doi: 10.1016/0006-291x(73)91462-9. [DOI] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B., Sodja A. The amino acid sequence of an extracellular nuclease of Staphylococcus aureus. 3. Complete amino acid sequence. J Biol Chem. 1967 Oct 25;242(20):4752–4758. [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Effect of hydroxyurea on replication of bacteriophage T4 in Escherichia coli. J Virol. 1969 Mar;3(3):331–336. doi: 10.1128/jvi.3.3.331-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Hurwitz J. DNA replication in Escherichia coli made permeable by treatment with high sucrose. Biochem Biophys Res Commun. 1972 Apr 14;47(1):202–211. doi: 10.1016/s0006-291x(72)80029-9. [DOI] [PubMed] [Google Scholar]
- Wovcha M. G., Tomich P. K., Chiu C. S., Greenberg G. R. Direct participation of dCMP hydroxymethylase in synthesis of bacteriophage T4 DNA. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2196–2200. doi: 10.1073/pnas.70.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]



