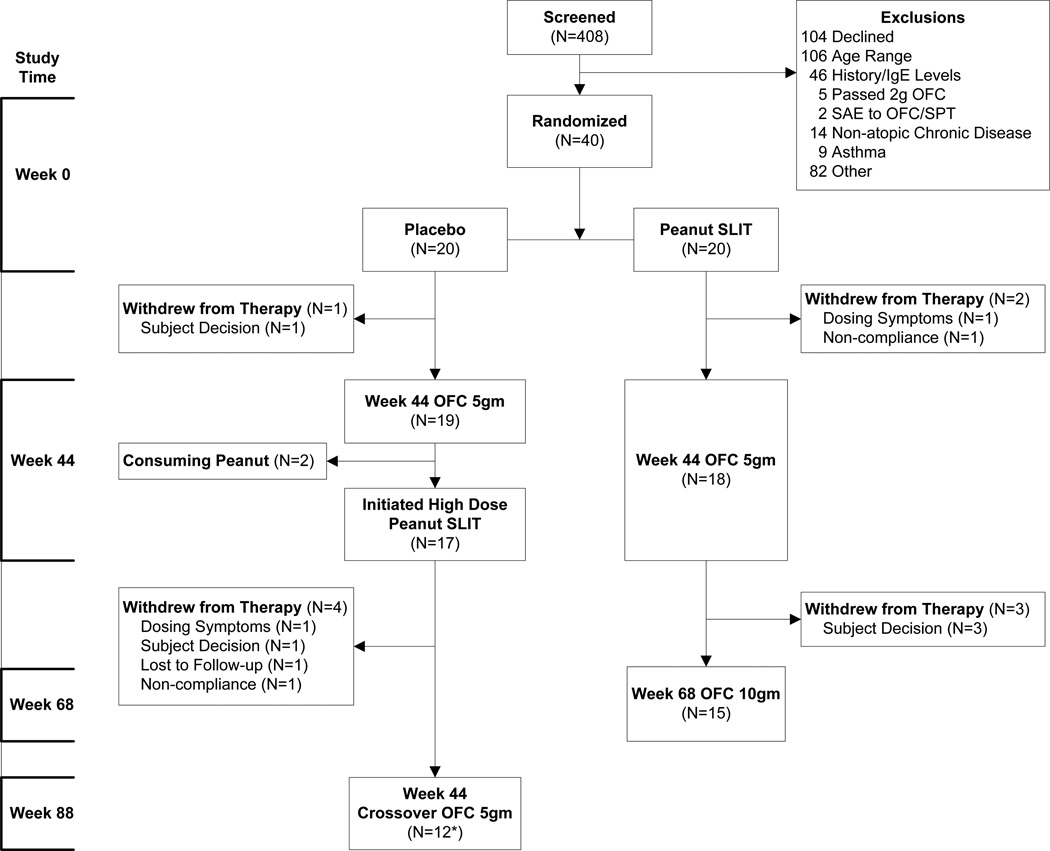
Figure 1. Enrollment and Disposition of Subjects.
The 40 subjects who passed screening were randomized onto placebo or peanut SLIT. After the Week 44 oral food challenge, the study was unblinded. Subjects in the original Peanut SLIT group continued on maintenance peanut SLIT therapy and received a Week 68 oral food challenge. Original placebo recipients were offered a higher dose peanut SLIT from week 44 to week 88, and then an oral food challenge.