Abstract
An efficient, rapid and improved in vitro plant regeneration protocol has been established for Withania somnifera L. using shoot tip and nodal explants, excised from 15 days old aseptic seedlings. A range of cytokinins were investigated for multiple shoot regeneration. Of the three cytokinins, 6-benzyladenine (BA), Kinetin (Kin) and 2-isopentenyl adenine (2-iP) evaluated as supplement to Murashige and Skoog (MS) medium, BA at an optimal concentration of 2.5 μM was most effective in proliferating apical and axillary buds. The highest regeneration frequency (95 %) and number of shoots (36.1 ± 0.33) were obtained on MS medium fortified with BA (2.5 μM) and NAA (0.5 μM) from nodal segments. High frequency of rooting (100 %) was obtained in in vitro raised shoots when transferred to half-strength MS medium supplemented with NAA (0.5 μM). Histological sections revealed that additional shoot bud primordia were differentiated within the explants just underneath the suberized cells which appeared to be arrested in their development. The presence of additional bud primordia within the explants is thereby helpful to maximize the potential of this system. The regenerated plantlets with well developed shoots and roots were hardened successfully, established in earthen pots containing garden soil and maintained in greenhouse with 95 % survival rate.
Keywords: Withania somnifera, Multiple shoots, Shoot tips, Nodal explants, Plant growth regulators, Histology
Introduction
Withania somnifera L. (Solanaceae) Dunal. commonly known as Ashwagandha is a valuable medicinal plant that has been used in Ayurvedic and Indigenous medicines for over 3,000 years (Anonymous 1976). It is a reputed medicinal shrub, widely distributed throughout the dry regions of India upto an altitude of 2,000 m in Himalayas. Ashwagandha roots are a constituent of over 200 formulations in Ayurveda, Siddha and Unani medicines (Asthana and Raina 1989; Singh and Kumar 1998). The leaves contain Withanolides like Withaferin-A that exhibit anti-bacterial and anti-tumor properties (Kurup 1956; Uma Devi et al. 1993). Its root contain a number of alkaloids like somniferine, withasomnine etc. which are prescribed for female disorders, cough, rheumatism, dropsy, arthritis, sedative for senile debility and also inhibits Alzheimer’s disease (Majumdar 1955; Kirtikar and Basu 1975; Nigam and Kandalkar 1995). W. somnifera appears in WHO Monographs on Selected Medicinal Plants and an American Herbal Pharmacopoeia is also forthcoming (Marderosion 2001).
The genetic diversity of W. Somnifera in India is now getting endangered (Antonisamy and Manickam 1999) at an alarming rate; because of ruinous harvesting practices for the production of medicines and a reduced span of viability and also low germination rate, restricts its propagation through seeds. Hence, there is a strong need for proactive understanding of the propagation, conservation and sustainable usage of W. somnifera clones within a reasonable time frame. Presently, micropropagation technique is especially being used not only for those plants which are difficult to be propagated through conventional practices, but also for the mass multiplication of existing stocks of germplasm for more biomass energy production and conservation of important, elite and rare plant species that are threatened or on the verge of extinction (Pandey et al. 1993; Faisal and Anis 2005; Faisal et al. 2005a, b; Anis et al. 2009). Further in vitro propagation through shoot tip and axillary bud culture is an easy and economic way for obtaining large number of consistently uniform and true-to-type plants (Hu and Wang 1983; Georg 1993). Although earlier attempts have been made for the propagation of W. somnifera through tissue culture (Kulkarni et al. 1999, 2000; Manickam et al. 2000) but considerable effort is still required to make it more practicable.
Histological analysis indicated that new meristem is formed closed to the exposed surface of the explants which interacts with the severed vascular bundles bringing about shoot formation. These developing buds always had the usual dome-shaped meristems surrounded by 2 leafy primordia which are connected with the vascular system. The histological studies also revealed that organogenesis often involves more than one cell that act in a coordinate manner (Brown and Thorp 1986).
The present study deals with the comparative performances of auxin-cytokinin singly or in various combinations on the induction and multiplication of axillary shoot buds from nodal and shoot tip explants to ensure high yield of biomass, continuous propagation followed by successful restoration of micropropagated plants into field conditions. The histological events leading to the organ formation were also evaluated.
Materials and methods
Establishment of aseptic seedlings and explants preparation
Seeds of W. somnifera were washed thoroughly under running tap water for 30 min, treated with 5 % (v/v) labolene for 10 min, and then rinsed with double distilled water. For surface disinfectation, the seeds were immersed for 4 min in 0.1 % (w/v) HgCl2 and finally rinsed three times with double distilled water and placed on ½ MS (Murashige and Skoog 1962) medium for germination. Nodal segments and shoot tips excised from 15 day old aseptic seedlings were used as explants.
Media and culture conditions
A culture medium containing MS salts supplemented with macro and micro-elements, sucrose 3 % (w/v) as carbon source and agar 0.8 % (w/v) as gelling agent was used for all cultures. The pH of the medium was adjusted to 5.8 before autoclaving at 121 °C (15 lbs) for 20 min. All the cultures were incubated in a growth room under controlled environmental conditions with a light intensity of 50 μmol m−2 s−1 provided by cool white fluorescent lamp (40 W, Philips, India) with a photoperiod of 16/8 h at 25 ± 2 °C.
Shoot initiation and multiplication
MS medium supplemented with various concentrations of plant growth regulators viz. BA, Kin and 2-iP (0.0, 0.1, 0.5, 1.0, 2.5, 5.0 and 10 μM) either singly or in combination with NAA and IBA (0.1, 0.5, 1.0 and 2.0 μM) were used for proliferation and multiplication of shoots. Cultures were subcultured onto fresh media after every 3 weeks. The frequency of explants producing shoots, number of shoots per explants and shoot length were recorded after 8 weeks of culture.
Rooting of microshoot
In vitro raised shoots (4–5 cm) were excised and transferred to half and full strength MS medium supplemented with NAA (0.1, 0.5, 1.0 and 2.0 μM) for root induction. Data was recorded on percentage of rooting, number and length of roots after 4 weeks of transferring to rooting media.
Acclimatization and transfer of plantlets to soil
Plantlets with well developed shoots and roots were removed from the culture vessels, washed thoroughly with tap water and transferred to different planting substrates viz. garden soil: vermicompost (3:1), SoilriteTM (Keltech Energies Ltd, Bangalore, India) and vermicompost under diffuse light (16/8 h photoperiod). Potted plantlets were covered with transparent plastic bags to ensure high humidity and watered every 3 day with half-strength MS salts without vitamins and sucrose for 2 weeks. Plastic bags were opened after 2 weeks in order to acclimatize plantlets to field conditions. After 4 weeks, acclimatized plants were transferred to pots containing normal garden soil and maintained in greenhouse.
Histology
For histological examination, cotyledonary node explants bearing regenerated shoot buds were fixed in 5:5:90 (v/v/v) formalin: acetic acid: ethanol (FAA) for 24 h and stored in 70 % (v/v) ethanol. Tissues were hydrated with a graded ethanol-xylol series followed by paraffin embedding using the method described by Johansen (1940). Longitudinal sections, 10 μm thick, were cut using a Spencer 820 rotatory microtome (American Optical Corporation, Baffalo, NY, USA) and the resulting paraffin ribbons were passed through a series of deparaffinising solutions, followed by staining with saffranin and fast green (Johansen 1940). Sections were examined under a light microscope (Olympus CH20i; India Pvt., Ltd., New Delhi).
Statistical analysis
All the experiment were conducted with a minimum of 20 replicate per treatment and repeated three times. The data was analysed statistically using SPSS ver. 16 (SPSS Inc. Chicago, USA). The significance of differences among means was found using Duncan’s multiple range test (DMRT) at P ≤ 0.05. The results are expressed as the means±SE of three experiments.
Results
Shoot regeneration from nodal explants
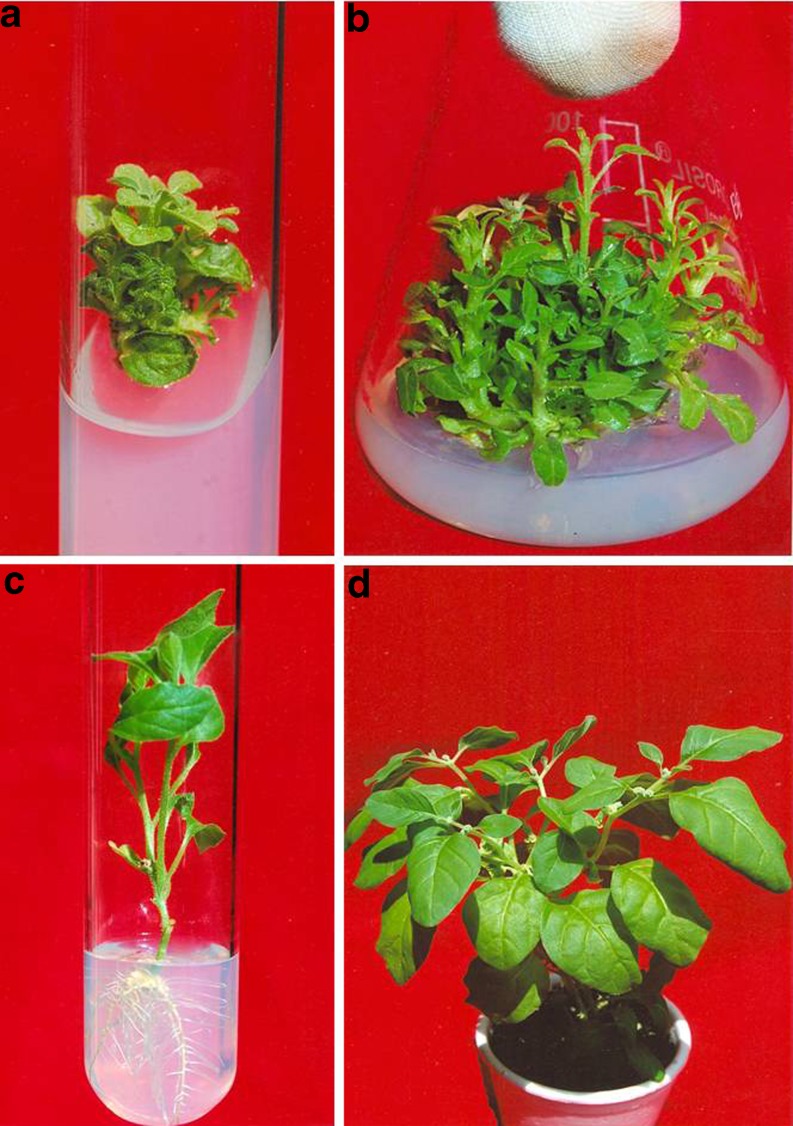
The morphogenetic responses of nodal explants evaluated to various cytokinins (BA, Kin & 2iP) at different concentrations (0.1–10.0 μM) showed a marked effect on multiple shoot induction (Table 1). Nodal explants cultured on MS basal medium served as control, did not promote axillary bud initiation and eventually necrosed, whereas, the presence of growth regulators favoured axillary bud induction and considerable enhancement in response was achieved with the application of different plant growth regulators (PGR’s). Of the three different cytokinins tested, BA was found to be more efficient than other cytokinins with respect to initiation and subsequent proliferation of shoots (Table 1). Multiple shoot buds were induced within 2–3 weeks of culture and the maximum frequency (95 %) was observed on MS medium supplemented with BA (2.5 μM) which induced (24.8 ± 2.33) shoots with a shoot length of (5.86 ± 0.52 cm) after 8 weeks of culture (Fig. 1a) (Table 1).
Table 1.
Effect of plant growth regulators on shoot regeneration from nodal explants of W. somnifera after 8 weeks of culture
| Plant growth regulators (μM) | Regeneration (%) | Mean number of shoots/explant | Mean shoot length (cm) | ||||
|---|---|---|---|---|---|---|---|
| BA | Kin | 2-iP | NAA | IBA | |||
| 0.0 | 00 | 0.00 ± 0.00l | 0.00 ± 0.00l | ||||
| 0.1 | 72 | 5.50 ± 0.76ijk | 1.26 ± 0.23ijk | ||||
| 0.5 | 75 | 9.50 ± 0.76gh | 2.33 ± 0.16hi | ||||
| 1.0 | 90 | 17.83 ± 1.48b | 3.26 ± 0.62fgh | ||||
| 2.5 | 95 | 24.8 ± 2.33c | 5.86 ± 0.52ab | ||||
| 5.0 | 85 | 16.8 ± 1.58de | 3.50 ± 0.28fg | ||||
| 10.0 | 70 | 8.33 ± 0.16gh | 1.26 ± 0.39ijk | ||||
| 0.1 | 63 | 4.33 ± 0.16jk | 1.13 ± 0.06jk | ||||
| 0.5 | 70 | 6.83 ± 0.33hij | 1.63 ± 0.88ijk | ||||
| 1.0 | 75 | 12.5 ± 0.00f | 2.16 ± 0.88hi | ||||
| 2.5 | 85 | 17.6 ± 2.58de | 4.66 ± 0.44cde | ||||
| 5.0 | 80 | 15.0 ± 0.00ef | 2.83 ± 0.66gh | ||||
| 10.0 | 75 | 9.50 ± 0.76gh | 1.50 ± 0.00ijk | ||||
| 0.1 | 60 | 3.16 ± 0.66k | 1.00 ± 0.00kl | ||||
| 0.5 | 70 | 5.00 ± 0.28ijk | 1.26 ± 0.23jk | ||||
| 1.0 | 75 | 7.66 ± 0.60ghi | 1.66 ± 0.16ijk | ||||
| 2.5 | 80 | 13.5 ± 0.00f | 3.50 ± 0.57fg | ||||
| 5.0 | 70 | 9.66 ± 0.60g | 2.33 ± 0.44hi | ||||
| 10.0 | 65 | 4.33 ± 0.16jk | 0.96 ± 0.23kl | ||||
| 2.5 | 0.1 | 85 | 28.3 ± 0.16b | 5.40 ± 0.10abcd | |||
| 2.5 | 0.5 | 95 | 36.1 ± 0.33a | 6.16 ± 0.33a | |||
| 2.5 | 1.0 | 85 | 22.3 ± 0.16a | 4.96 ± 0.08bcde | |||
| 2.5 | 2.0 | 80 | 18.3 ± 0.16d | 4.33 ± 0.24def | |||
| 2.5 | 0.1 | 75 | 16.3 ± 0.16de | 4.93 ± 0.13bcde | |||
| 2.5 | 0.5 | 90 | 22.3 ± 0.16c | 5.80 ± 0.00ab | |||
| 2.5 | 1.0 | 80 | 16.1 ± 0.16de | 5.53 ± 0.17abc | |||
| 2.5 | 2.0 | 75 | 12.3 ± 0.16f | 4.06 ± 0.06ef | |||
Data represent mean±SE. Means sharing the same letter within columns are not significantly different (P = 0.05) using Duncan’s multiple range test (DMRT)
Fig. 1.
a Multiple shoot induction from nodal explants on MS medium amended with BA (2.5 μM), 8 week old culture. b Shoot elongation and multiplication on MS medium amended with BA (2.5 μM)+NAA (0.5 μM), 8 week old culture. c Rooting of in vitro microshoots on ½ Ms+NAA (0.5 μM), 4 week old culture. d An acclimatized plant, 4 week old
MS medium supplemented with Kin (2.5 μM) induced (17.6 ± 2.58) shoots with a shoot length of (4.66 ± 0.44 cm), while 2-iP at the same concentration produced (13.5 ± 0.00) shoots with shoot length of (3.50 ± 0.57 cm) per explants after 8 weeks of cultures (Table 1). Further increase in the concentration beyond the optimal level did not improve the parameters and suppressed regeneration frequency, number of shoot and shoot length. Slight basal callusing was also observed which was removed during subculturing as precautionary measures, as callus formation retarded the shoot multiplication rate.
The combined effect of various auxins (NAA & IBA) at different concentration (0.1, 0.5, 1.0 and 2.0 μM) with the optimal concentrations of BA (2.5 μM) was also evaluated for enhanced multiplication rate. Among all the treatment tried, MS medium supplemented with BA (2.5 μM) and NAA (0.5 μM) gave maximum percentage response (95 %), number of shoots (36.1 ± 0.33) with shoot length of (6.16 ± 0.33 cm) per nodal segment after 8 weeks of culture (Fig. 1b) (Table 1). The percentage and number of shoots per explants increased with an increase in concentration but only upto 0.5 μM NAA. However, a gradual decrease in the number of shoots per explants was observed at lower (0.1 μM) or higher concentrations of NAA (1.0 & 2.0 μM) (Table 1). The medium supplemented with BA (2.5 μM) and IBA (0.1–2.0 μM) combination was less effective than BA and NAA for the proliferation and multiplication of shoots (Table 1).
Shoot regeneration from shoot tip explants
The shoot tip explants on PGRs free medium (control) did not exhibit shoot bud differentiation even after 4 weeks of incubation, turned brown and finally died. Shoot tips cultured on MS medium supplemented with different cytokinins viz. BA, Kin and 2-iP with various concentrations (0.1–10.0 μM) exhibited multiple shoot formation with varied frequency (Table 2). The maximum regeneration frequency (92 %), mean number of shoots (18.0 ± 2.25) with shoot length of (4.00 ± 0.50 cm) was found on MS medium supplemented with BA (2.5 μM) after 8 weeks of culture (Table 2). BA was found to be superior cytokinin for apical bud induction and subsequent proliferation of shoots. Although MS medium containing Kin (2.5 μM) formed (14.3 ± 0.16) shoots with shoot length (3.66 ± 0.29 cm), while, 2-iP at the same concentration formed about (11.1 ± 0.16) shoots with a shoot length of (3.50 ± 0.00 cm) after 8 weeks of culture (Table 2).
Table 2.
Effect of plant growth regulators on shoot regeneration from shoot tip explants of W. somnifera after 8 weeks of culture
| Plant growth regulators (μM) | Regeneration (%) | Mean number of shoots/explant | Mean shoot length (cm) | ||||
|---|---|---|---|---|---|---|---|
| BA | Kin | 2-iP | NAA | IBA | |||
| 0.0 | 00 | 0.00 ± 0.00m | 0.00 ± 0.00m | ||||
| 0.1 | 70 | 4.33 ± 0.16l | 1.90 ± 0.20l | ||||
| 0.5 | 85 | 7.66 ± 0.60jk | 2.10 ± 0.20ghi | ||||
| 1.0 | 90 | 15.1 ± 0.16ef | 3.16 ± 0.16ef | ||||
| 2.5 | 92 | 18.0 ± 2.25d | 4.00 ± 0.50cd | ||||
| 5.0 | 80 | 12.3 ± 0.16g | 2.43 ± 0.23g | ||||
| 10.0 | 70 | 8.16 ± 0.16jk | 1.50 ± 0.17ijkl | ||||
| 0.1 | 70 | 2.83 ± 0.33l | 1.06 ± 0.06kl | ||||
| 0.5 | 72 | 6.83 ± 0.33jk | 1.66 ± 0.06hijk | ||||
| 1.0 | 80 | 12.3 ± 0.16g | 2.00 ± 0.11ghi | ||||
| 2.5 | 75 | 14.3 ± 0.16f | 3.36 ± 0.29eh | ||||
| 5.0 | 70 | 10.1 ± 0.16hi | 1.96 ± 0.39gij | ||||
| 10.0 | 60 | 7.33 ± 0.16jk | 1.46 ± 0.17ijkl | ||||
| 0.1 | 70 | 2.83 ± 0.33l | l0.90 ± 0.20l | ||||
| 0.5 | 70 | 4.33 ± 0.16l | 1.33 ± 0.13jkl | ||||
| 1.0 | 75 | 8.00 ± 0.28jk | 2.56 ± 0.12fg | ||||
| 2.5 | 80 | 11.1 ± 0.16gh | 3.50 ± 0.00de | ||||
| 5.0 | 75 | 8.83 ± 0.16ij | 2.16 ± 0.16gh | ||||
| 10.0 | 65 | 4.16 ± 0.16l | 1.26 ± 0.16kl | ||||
| 2.5 | 0.1 | 85 | 24.4 ± 0.58b | 4.10 ± 0.37cd | |||
| 2.5 | 0.5 | 90 | 32.0 ± 0.29a | 6.03 ± 0.12a | |||
| 2.5 | 1.0 | 85 | 20.2 ± 0.14c | 4.40 ± 0.10c | |||
| 2.5 | 2.0 | 80 | 15.3 ± 0.23ef | 3.26 ± 0.12e | |||
| 2.5 | 0.1 | 75 | 12.2 ± 0.15g | 4.26 ± 0.80c | |||
| 2.5 | 0.5 | 80 | 21.2 ± 0.12c | 5.43 ± 0.23b | |||
| 2.5 | 1.0 | 80 | 16.3 ± 0.23e | 4.20 ± 0.05c | |||
| 2.5 | 2.0 | 70 | 10.3 ± 0.25hi | 3.10 ± 0.10ef | |||
Data represent mean±SE. Means sharing the same letter within columns are not significantly different (P = 0.05) using Duncan’s multiple range test (DMRT)
The efficiency of shoot bud induction from shoot tip explants was also evaluated on MS medium supplemented with the optimal concentration of BA (2.5 μM) along with different auxins (NAA or IBA) with various concentrations (0.1–2.0 μM) (Table 2). The optimal concentration of BA (2.5 μM) with NAA (0.5 μM) gave maximum regeneration frequency (95 %) with mean number of shoots (32.0 ± 0.29) and shoot length (6.03 ± 0.12 cm) per explants after 8 weeks of culture (Table 2).
In vitro rooting and acclimatization
The in vitro regenerated microshoots (4–5 cm) were excised aseptically and transferred to MS or ½ MS medium with different concentrations of NAA (0.1–2.0 μM) (Table 3). The maximum rooting percentage (100 %), highest number (18 ± 0.00) of roots with root length (3.22 ± 0.24 cm) per shoot was obtained on half strength MS medium supplemented with NAA (0.5 μM) (Fig. 1c) (Table 3). More than 350 plantlets with 4–6 fully developed leaves and well developed root system were excised, successfully hardened off inside the growth room in selected planting substrate for 4 weeks (Fig. 1d) and eventually in normal garden soil.
Table 3.
Effect of MS strength and NAA concentrations on root induction from in vitro raised shoots of W. somnifera after 4 weeks of culture
| Treatments (μM) | Root induction (%) | Mean number of roots/shoot | Mean root length (cm) |
|---|---|---|---|
| MS+NAA (0.1) | 75 | 8.83 ± 0.16e | 3.40 ± 0.10c |
| MS+NAA (0.5) | 85 | 12.8 ± 0.33c | 3.66 ± 0.46bc |
| MS+NAA (1.0) | 80 | 6.46 ± 0.14f | 3.16 ± 0.03c |
| MS+NAA (2.0) | 70 | 5.33 ± 0.16g | 2.50 ± 0.02d |
| ½ MS+NAA (0.1) | 90 | 12.8 ± 0.33c | 3.20 ± 0.15c |
| ½ MS+NAA (0.5) | 100 | 18.5 ± 0.00a | 5.33 ± 0.24a |
| ½ MS+NAA (1.0) | 95 | 15.2 ± 0.17b | 4.10 ± 0.50b |
| ½ MS+NAA (2.0) | 80 | 10.2 ± 0.11d | 3.05 ± 0.05cd |
Data represent mean±SE. Means sharing the same letter within columns are not significantly different (P = 0.05) using Duncan’s multiple range test (DMRT)
Histological analysis
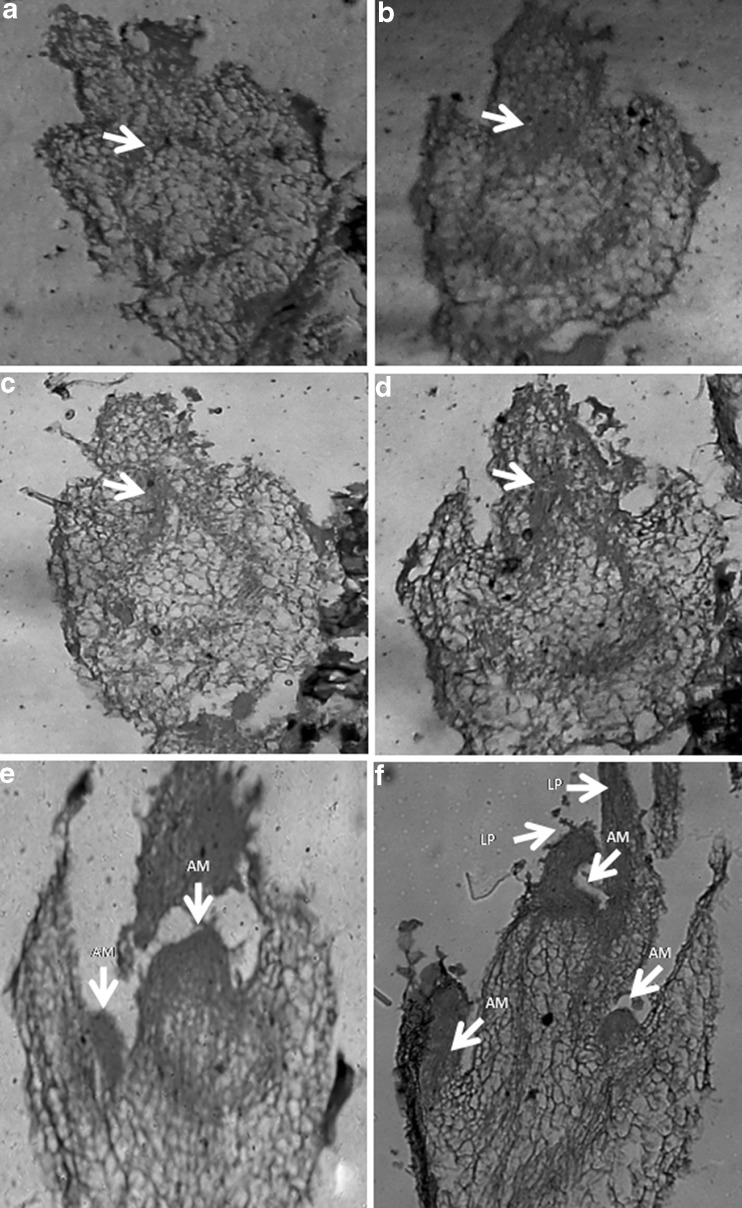
Histological sections showed spherical dense cell clusters of meristematic cells with a separate ring-shaped vascular system (Fig. 2a). The meristematic cells begin to divide and differentiate into shoot meristem initials. During the course of bud formation, the vascular ring expanded towards the periphery (Fig. 2b & c), supplying the vascular connection to the differentiating shoot buds (Fig. 2d). Further, differentiation of the adjoining cell layers results in the formation of multiple shoot bud with well developed apical meristem and leaf primordia (Fig. 2e). The shoot meristem give rise to leaf primordia and protrudes above the surface of each responding explants (Fig. 2f).
Fig. 2.
a Transverse section of axillary bud, arrow indicating ring shaped vascular system. b T.S of shoot bud, arrow indicating the expansion of vascular ring towards the periphery. c T.S of shoot bud, arrow indicating the origin of shoots bud primordia from the peripheral layer. d T.S showing apical meristem with leaf primordia. e Longitudinal section of bud showing differentiation of multiple shoot buds. f Shoot buds showing cyto-histological zones with well developed apical dome and leaf primordia. Arrows in Fig. (a–f): AM apical meristem, LP leaf primordial
Discussion
Varied morphogenetic responses with different concentrations and combinations of plant growth regulators were recorded from nodal and shoot tip explants. Effect of various PGRs on multiple shoot induction from apical and axillary buds have been reported in many medicinal plants like Ocimum sanctum (Begum et al. 2000); Wedelia calendulaceae (Emmanuel et al. 2000); Psoralea corylifolia (Anis and Faisal 2005); Heliotropium indicum L. (Kumar and Rao 2007); Balanites aegyptiaca (Siddique and Anis 2009); Cardiospermum halicacabum L. (Jahan and Anis 2009). Shoot buds developed precociously from nodal segments and shoot tip explants cultured on MS basal medium supplemented with different cytokinins. These buds proliferated to form clusters of secondary and tertiary shoots. Among the different cytokinins tested, maximum shoot bud induction and shoot sprouting was obtained on MS medium supplemented with BA (2.5 μM) from both nodal segments and shoot tip explants, followed by Kin and 2-iP. The superiority of BA over other cytokinins in a tissue culture system has been well demonstrated in a number of precocious studies using a variety of explants like Cucumis sativus (Ahmad and Anis 2005); Adhatoda vasica (Abhyankar and Reddy 2007); Ocimum basilicum (Siddique and Anis 2008); Gynura procumbens (Keng et al. 2009); Vitex negundo (Ahmad and Anis 2010). The naturally occurring ribosides and nucleotides in the cytokinin BA are relatively more stable in comparison to other cytokinins (Letham and Palni 1983), one of the possible explanations for the improved response obtained. There was a linear correlation of BA upto an optimal level and the number of shoots per explants. In contrast, higher concentration of BA beyond the optimal level (2.5 μM) inhibited the overall shoot sprouting frequency, number of shoot and shoot length. Reduction in the regeneration potential appeared to be due to detrimental effect of high concentration on the cells predetermined to form vegetative buds. Reduction in shoot number at concentration higher than the optimal level has been reported in various medicinal plants including Ruta graveolens (Faisal et al. 2005b); Eclipta alba (Hussain and Anis 2006) and Tylophora indica (Faisal et al. 2007). A comparison of relative effectiveness of different cytokinins for multiple shoot formation revealed the order of effectiveness as BA>Kin>2-iP.
A lower concentration of auxin along with a higher concentration of cytokinins was most promising for the induction and multiplication of shoots in W. somnifera from both the explants. One of the advantages of adding auxin at lower concentration on the culture media is to nullify the effect of the higher concentration of cytokinin on axillary shoot elongation (Hu and Wang 1983). Addition of NAA markedly enhanced the percent regeneration, number of shoots and shoot length, whereas; IBA did not significantly improve the parameters evaluated. Among all the treatments tested, the maximum percentage regeneration (95 %) and (90 %) with the highest (36.1 ± 0.33) and (32.0 ± 0.29) number of shoots were obtained at 2.5 μM BA and 0.5 μM NAA from nodal segments and shoot tip explants, respectively. The potential combination of BA and NAA for in vitro shoot induction and multiplication has been reported in various medicinal plants such as Centella asiatica (Tiwari et al. 2000); Rauvolfia tetraphylla (Faisal and Anis 2002); Psoralea corylifolia (Anis and Faisal 2005); Asteracantha longifolia (Panigrahi et al. 2006); Eclipta alba (Hussain and Anis 2006). The present protocol reports the simplest and one step method for mass multiplication of shoots and also minimizes the additional step for the shoot elongation in Withania somnifera as repoted by Sivanesan and Murugesan 2008 and Aniel et al. 2011.
In the present study, the highest bud proliferation was observed in axillary bud explants within 8 weeks of culture and found to be better as compared to shoot tip explants for the induction and proliferation of shoots. The axillary bud proliferation through in vitro technique is reported to be the safest and faithful strategy which maintains genetic integrity of developing progenies (Salvi et al. 2002).
During micropropagation, rooting in microshoots is often problematic and losses at this stage have vast economic consequences (De Klerk 2002). Adventitious root formation is fascinating scientific subject matter as many genotypes are recalcitrant to root (Hartmann et al. 1990). Adventitious rooting has been considered and manipulated as a single phase process in which auxin is reported to play major role. In the present study, ½ MS medium augmented with NAA (0.5 μM) was found better than IBA in stimulating adventitious root formation (Table 3). The effectiveness of NAA in rooting of in vitro regenerated shoots has been well documented in plants like Cucumis sativus (Ahmad and Anis 2005). Planting substrate (garden soil, soilrite & vermicompost) also plays an important role in acclimatization of plantlets and the highest survival rate (95 %) was obtained in soilrite (Table 4). There was no detectable variation among the potted plants with respect to morphological and growth characteristics.
Table 4.
Evaluation of different planting substrates for hardening off in vitro raised plantlets of W. somnifera after 4 weeks of transfer
| Planting Substrate | No. of plants transferred | No. of surviving plants | % Survival |
|---|---|---|---|
| Vermicompost | 50 | 35 | 73.6 ± 1.83c |
| SoilriteTM | 50 | 45 | 95.0 ± 0.50a |
| Garden soil: vermicompost (3:1) | 50 | 40 | 90.3 ± 0.33b |
Data represent mean±SE. Means sharing the same letter within columns are not significantly different (P = 0.05) using Duncan’s multiple range test (DMRT)
In most plants, shoot bud primordia developed in vitro; contain all the various components of normal tissue. Shoot bud primordia are initiated from meristem cells in the region of the severed vascular bundles near the exposed surface of the explants. Shoot meristems are always formed in association with the vascular tissue, it is likely that the material such as additional nutrients or metabolites may be present in or near the vascular bundles which could induce shoot organogenesis from the meristematic cells (Miranda et al. 1999). Furthermore, it is important to note that bud primordia are formed away from the surface, and hence, they have an endogenous origin. The presence of several shoot bud primordia confirmed the buds to be originated from the meristematic structures. Direct shoot formation on explants tissue is commenced by cell division and formation of meristematic primordia comprising numerous cells. Direct regeneration of shoot buds may always be formed from one or few cells that originate from single cell as reported by Broertjes and Keen (1980).
Cellular differentiation and organogenesis in tissue and organ cultures have been found to be controlled by an interaction between cytokinins and auxin concentrations. It is apparent that not only auxins and cytokinin levels but the proportions of one to other are determinants for cell cycle, cell division and differentiation control. Cell division seems to be regulated by the joint action of auxins-cytokinins, each of which appears to influence different phases of cell cycle. Auxin exerts an effect on DNA replication, while cytokinin seems to exert some control over the events leading to mitosis (Pasternak et al. 2000). Therefore, auxins might be considered as “inducers” of the all cycle while cytokinins might behave more as its “promoter” (Wood et al. 1990). Normal cell division requires synchrony between the S phase and cell division, suggesting that auxin and cytokinin levels in cultures need to be carefully matched. Cells are thought not to enter mitosis unless cytokinin is present.
In the present study, the histological examination showed that most of the shoot buds revealed visible connection with the original vascular tissue and originated from mersistematic zone beneath the epidermis. Similar results were also reported in Cyamopsis tetragonoloba L. (Ahmad and Anis 2007).
Conclusion
The present protocol describes an improved and a successful tissue culture system of W. somnifera from shoot tip and nodal explants. The study also proves the potential of explants for direct organogenesis without an intervening callus phase, provides a model system for studying the basic development and morphology of the plant. The micropropagation system has assured effective establishment, multiplication, rhizogenesis and acclimatization of the species year round, irrespective of seasonal constraints. It could support domestication/conservation of plant species from indiscriminate exploitation from its natural resources, ultimately enabled to keep pace with commercial needs. The in vitro culture strategies developed here could be worked as useful tool to increase the biomass and yield of pharmaceutically important active principles (withanolides, somniferine etc.) accumulated in W. somnifera cultures.
Acknowledgements
Research support provided by the Department of Science and Technology (DST) and University Grants Commission (UGC) Govt. of India, New Delhi, in the form of DST-FIST (2005) and UGC-SAP (2009) Programmes is duly acknowledged.
Abbreviations
- BA
6-benzyladenine
- IBA
Indole-3-butyric acid
- Kin
Kinetin
- NAA
α-naphthalene acetic acid
- MS
Murashige and Skoog’s medium
- 2-iP
2-isopentenyl adenine
- NS
Nodal segment
- ST
Shoot tip segment
References
- Abhyankar G, Reddy VD. Rapid micropropagation via axillary bud proliferation of Adhatoda vasica Nees from nodal segments. Indian J Exp Biol. 2007;45:286–271. [PubMed] [Google Scholar]
- Ahmad N, Anis M. In vitro mass propagation of Cucumis sativus L. from nodal segments. Turk J Bot. 2005;29:237–240. [Google Scholar]
- Ahmad N, Anis M. Rapid plant regeneration protocol for cluster bean (Cyamopsis tetragonoloba L. Taub.) J Hortic Sci Biotechnol. 2007;82:585–589. [Google Scholar]
- Ahmad N, Anis M. An efficient in vitro process for recurrent production of cloned plants of Vitex negundo L. Eur J For Res. 2010;130:135–144. doi: 10.1007/s10342-010-0415-y. [DOI] [Google Scholar]
- Aniel KO, Jyothirmayee G, Subba TS. Multiple shoot regeneration from nodal explants of Ashwagandha (Withania somnifera (L.) Dunal. Asian J Exp Biol Sci. 2011;2:636–640. [Google Scholar]
- Anis M, Faisal M. In vitro regeneration and mass multiplication of Psoralea corliyfolia- an endangered medicinal plant. Indian J Biotechnol. 2005;4:261–264. [Google Scholar]
- Anis M, Husain MK, Faisal M, Shahzad A, Ahmad N, Siddique I, Khan H. In vitro approaches for plant regeneration and conservation of some medicinal plants. In: Kumar A, Sopory SK, editors. Recent advances in plant biotechnology & its application. New Delhi: IK International P. Ltd.; 2009. pp. 397–410. [Google Scholar]
- Anonymous (1976) In the wealth of India (Raw Materials). CSIR, New Delhi, India 10:580–595
- Antonisamy R, Manickam VS (1999) Conservation through micropropagration and restoration of selected rare and endangered medicinal plants of South India, XVI Int. Bot Congr. held in 1–7 Aug. Saint Louis, Missouri, USA
- Asthana R, Raina MK. Pharmacology of Withania somnifera (L.) Dunal- a review. Indian Drugs. 1989;26:199–205. [Google Scholar]
- Begum F, Amin N, Azad AK. In Vitro clonal propagation of holy basil- Ocimum sanctum L. Plant Tissue Cult. 2000;10:31–37. [Google Scholar]
- Broertjes C, Keen A. Adventitious buds. Do they develop from one cell? Euphytica. 1980;29:73–87. doi: 10.1007/BF00037251. [DOI] [Google Scholar]
- Brown DCW, Thorp TA (1986) Plant regeneration by organogenesis. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants. Academic Press, Orlando 3:49–66
- Klerk D. Rooting of microshoots: theory and practices. Vitro Cell Dev Biol Plant. 2002;38:415–422. doi: 10.1079/IVP2002335. [DOI] [Google Scholar]
- Emmanuel S, Ignacimuthu S, Kathiravan K. Microproapagation of Wedelia calendulacea L. -a medicinal plant. Phytomorphology. 2000;50:195–200. [Google Scholar]
- Faisal M, Anis M. Rapid in vitro propagation of Rauvolfia tetraphylla L.- an endangered medicinal plant. Physiol Mol Biol Plants. 2002;8:295–299. [Google Scholar]
- Faisal M, Anis M. An efficient in vitro method for mass propagation of Tylophora indica. Biol Plant. 2005;49:1478–1480. doi: 10.1007/s10535-005-7260-8. [DOI] [Google Scholar]
- Faisal M, Ahmad N, Anis M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult. 2005;80:187–190. doi: 10.1007/s11240-004-0567-x. [DOI] [Google Scholar]
- Faisal M, Ahmad N, Anis M. In vitro regeneration and mass propagation of Ruta graveolens L.—a multipurpose shrub. Hortic Sci. 2005;40:1478–1480. [Google Scholar]
- Faisal M, Ahmad N, Anis M. An efficient micropropagation system for Tylophora indica: an endangered, medicinally important plant. Plant Biotechnol Rep. 2007;1:155–161. doi: 10.1007/s11816-007-0025-4. [DOI] [Google Scholar]
- Georg EF. Plant propagation by tissue culture, part-I. The technology. Basingstoke: Exegetics Ltd; 1993. [Google Scholar]
- Hartmann HT, Kester DE, Davies FT., Jr . Plant propagation priciples and practices. 5. Englewood Chifts: Prentice Hall; 1990. [Google Scholar]
- Hu CY, Wang PJ. Meristem shoot tip and bud culture. In: Evans DA, Sharp WR, Ammirato PV, Yamada Y, editors. Hand book of plant tissue culture. New York: MacMillan Publ. Co.; 1983. pp. 127–227. [Google Scholar]
- Hussain MK, Anis M. Rapid in vitro propagation of Eclipta alba (L.) Hassk. through high frequency axillary shoot proliferation. Acta Physiol Plant. 2006;28:25–330. [Google Scholar]
- Jahan AA, Anis M. In Vitro rapid multiplication and propagation of Cardiospermum halicacabum L. through axillary bud culture. Acta Physiol Plant. 2009;31:133–138. doi: 10.1007/s11738-008-0211-1. [DOI] [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill; 1940. pp. 126–154. [Google Scholar]
- Keng CL, Yee LS, Pin PL. Micropropagation of Gynura procumbens (Lour.0 Merr. an important medicinal plant. J Med Plant Res. 2009;3:105–111. [Google Scholar]
- Kirtikar KR, Basu BD. Indian medicinal plants 3. 1. Dehradun: Bishen Singh Mahendra Pal Singh; 1975. pp. 1774–1777. [Google Scholar]
- Kulkarni AA, Thangane SR, Krishnamoorthy KV. Withania somnifera (L.) Dunal. In: Ravishankar GA, editor. Biotechnological application of plant tissue and cell culture. India: Oxford, IBH; 1999. pp. 156–159. [Google Scholar]
- Kulkarni AA, Thangane SR, Krishnamoorthy KV. Direct shoot regeneration from node, internode, hypocotyls and embryo explants of Withania somnifera L. Plant Cell Tissue Organ Cult. 2000;62:203–209. doi: 10.1023/A:1006413523677. [DOI] [Google Scholar]
- Kumar MS, Rao MV. In Vitro micropropagation of Heliotropium indicum L.-an ayurvedic herb. Indian J Biotechnol. 2007;6:245–249. [Google Scholar]
- Kurup PA. Antibiotic principle of the leaves of Withania somnifera. Curr Sci. 1956;25:57–58. [Google Scholar]
- Letham DS, Palni LMS. The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol. 1983;34:163–197. doi: 10.1146/annurev.pp.34.060183.001115. [DOI] [Google Scholar]
- Majumdar DN. Withania somnifera Dunal. Part II: Alkaloid constituents and their chemical characterization. Indian J Pharmacol. 1955;17:158–161. [Google Scholar]
- Manickam VS, Mathavan RE, Antonisamy R. Regeneration of Indian ginseng plantlets from stem callus. Plant Cell Tissue Organ Cult. 2000;62:181–185. doi: 10.1023/A:1006499522799. [DOI] [Google Scholar]
- Marderosion AD. The review of natural products. St. Louis: Facts and Comparisons; 2001. pp. 630–632. [Google Scholar]
- Miranda J, Koschuh MN, Yeung EC, Chinnappa CC. In Vitro plantlet regeneration from hypocotyls explants of Stellaria longipes (Caryophyllaceae) Can J Bot. 1999;77:318–322. doi: 10.1139/cjb-77-2-318. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nigam KB, Kandalkar VS (1995) Ashwagandha. In: Chadha KL, Gupta R (eds) Adv. In hortic. medicinal and aromatic plants. Malhotra Publishing House, New Delhi 11:337–344
- Pandey NK, Tewari KC, Tewari RN, Joshi GC, Pandey VN, Pandey G (1993) Medicinal plants of Komaon Himalaya, strategies for conservation. In: Dhar U (eds) Himalayan biodiversity conservation strategies 3:293–302
- Panigrahi J, Mishra RR, Behera M. In vitro multiplication of Asteracantha longifolia L. Nees- a medicinal herb. Indian J Biotechnol. 2006;5:562–564. [PubMed] [Google Scholar]
- Pasternak T, Miskolczi P, Ayaydin F, Meszaros T, Dudits D, Feher A. Exogenous auxin and cytokinin dependent activation of CDKs and cell division in leaf protoplast-derived cells of alfalfa. Plant Growth Regul. 2000;32:129–141. doi: 10.1023/A:1010793226030. [DOI] [Google Scholar]
- Salvi ND, George L, Eapen S. Micropropagation and field evaluation of micropropagated plants of turmeric. Plant Cell Tissue Organ Cult. 2002;68:143–151. doi: 10.1023/A:1013889119887. [DOI] [Google Scholar]
- Siddique I, Anis M. An improved plant regeneration system and ex vitro acclimatization of Ocimum basilicum L. Acta Physiol Plant. 2008;30:493–499. doi: 10.1007/s11738-008-0146-6. [DOI] [Google Scholar]
- Siddique I, Anis M. Direct plant regeneration from nodal explants of Balanites aegyptiaca L. (Del.): a valuable medicinal tree. New Forest. 2009;37:53–62. doi: 10.1007/s11056-008-9110-y. [DOI] [Google Scholar]
- Singh S, Kumar S. Withania somnifera: the Indian Ginseng Ashwagandha. Lucknow: Central Institute of Medicinal and Aromatic Plants; 1998. [Google Scholar]
- Sivanesan I, Murugesan K. An efficient regeneration from nodal explants of Withania somnifera L. (Dunal) Asian J Plant Sci. 2008;7:551–556. doi: 10.3923/ajps.2008.551.556. [DOI] [Google Scholar]
- Tiwari KN, Sharma NC, Tiwari V, Singh BD. Micropropagation of Centella asiaticah-avaluable medicinal herb. Plant Cell Tissue Organ Cult. 2000;63:179–185. doi: 10.1023/A:1010690603095. [DOI] [Google Scholar]
- Uma Devi P, Sharada AC, Solomon FE. Anti-tumor and radiosensitizing effects of Withania somnifera (Ashwagandha) on the transplantable mouse tumor sarcoma. Indian J Exp Biol. 1993;31:607–611. [PubMed] [Google Scholar]
- Wood HN, Sterner R, Alves LM, Basile DV. Auxin-phorbol ester: an example of two stage-stage initiation promotion system mediating cell proliferation in plants. Vitro Cell Dev Biol Plant. 1990;26:1125–1127. doi: 10.1007/BF02623688. [DOI] [Google Scholar]