Abstract
There has been a tremendous increase in global demand for marine and freshwater fish to meet the protein needs of our expanding human population. However, due to the limited capacity of the wild-capture sector and a levelling of production from capture fisheries, the practice of farming aquatic animals has expanded rapidly to become a major global industry. Aquaculture, particularly freshwater aquaculture is now integral to the economies of many countries. A large number of aquatic animal species are farmed in high density in freshwater, brackish and marine systems, where they are exposed to new environments and potentially new diseases. Further, environmental stress factors, the use of manufactured feeds, and prolific global trade has led to the emergence and spread of new diseases. Viral pathogens, established for decades or newly emerging as disease threats, are particularly challenging since there are few efficacious treatments. Vaccines have been developed for some viral fish pathogens in salmonids, but vaccines are not available for many of the viral pathogens important in Asia. Control and eradication programs are difficult because many viral infections remain latent until adverse environmental conditions, such as overcrowding or poor water quality, trigger the onset of disease. Here, we review the more significant viral pathogens of finfish in the Asia–Pacific including both those with a long history in Asian aquaculture and emerging pathogens including betanodaviruses and koi herpes virus that have caused massive losses in the freshwater aquaculture and ornamental fish industries.
Keywords: Virus, Fish, Asia, Aquaculture
Introduction
The aquaculture industry in Asia is growing rapidly, with the development of methods and markets for new species. Asia, particularly China, contributes significantly to global aquaculture production. More than 90 % of the world’s aquaculture production is coming from Asia. Further, the majority of aquaculture production of fish, crustaceans and molluscs continues to come from the freshwater environment (57.7 % by volume and 48.4 % by value) [6]. Wild fisheries yields have come to a plateau or declined as harvests exceed their sustainable capacity, so there is continuous demand for increased aquacultural production. The aquaculture industry is highly diverse in terms the species cultured, culture systems utilized, and culture intensity. Like other farming sectors, the aquaculture industry must also deal with diseases caused by varieties of pathogens.
The increase in the aquaculture industry world-wide has provided new opportunities for the transmission of aquatic viruses. The occurrence of viral diseases remains a significant constraint for aquaculture production and for the sustainability of biodiversity in the natural environment. The control of endemic diseases imposes severe continuing costs for producers. If we consider a single disease, white-spot syndrome disease of shrimp (WSS) has cost billions of dollars world-wide [30]. The elimination of disease outbreaks, such as infectious salmon anaemia (ISA) in Scotland in 1998/1999, required unexpected expenditures for both the industry and government. Emerging diseases also can have serious impacts on wild populations [25]. For example, crayfish plague, introduced from the USA, has impacted native crayfish populations over much of Europe [2].
In this review, we provide an overview of some of the significant viral pathogens affecting freshwater finfish species in Asia–Pacific. The diseases included in this review are those reported to the Network of Aquaculture Centre in Asia–Pacific (NACA) in their online quarterly aquatic animal disease report and other viral fish pathogens that are associated with fish health problems in the Asian–Pacific Region. The review does not intend to discuss various diagnostic techniques used for viral diseases of fish since these have become relatively standard and include pathological and histopathological examinations, isolation of virus in cell culture systems, molecular PCR-based techniques, in situ hybridization and various immunodiagnostic techniques.
Spring Viremia of Carp
Spring Viremia of Carp Virus (SVCV) produces disease in a diverse group of wild and cultured fishes including common carp, other cyprinids, pike (escocids) and even the wels catfish (Silurus glanis). Non-piscine carriers may include herons, leeches and parasitic copepods (Argulus sp.) [1]. The SVCV is classified as a member of the family Rhabdoviridae, belonging to genus Vesiculovirus. The genome is linear single-stranded negative sense RNA. SVC has been officially been reported from China and Iran and has a worldwide distribution in the northern hemisphere [5]. Zhang et al. [64] screened large numbers of ornamental fish and reported the existence of this virus in China. The disease primarily affects fish less than 1-year old. Infected fish show signs of exophthalmia, swollen abdomen, darkening of skin, petechial haemorrhages on skin and gill, swollen and protruded vent and loss of equilibrium. The mortality may range from 30 to 100 %. Grossly, the infected fish show haemorrhages in gills, abdominal tissue, swim bladder and other internal organs, and ascites.
The SVC virus causes a highly contagious disease among the common carp. The young fish are more susceptible to disease at water temperature up to 20 °C. The survivor of this infection acts as carrier of the virus. The virus enters the water through faeces, urine and spawning fluids as well as external mucous, skin secretion and infected eggs. Blood-sucking parasites such as copepods and leeches can transmit the virus from carp to carp [1].
Epizootic Haematopoietic Necrosis
Epizootic haematopoietic necrosis viris (EHNV) is an iridovirus (ranavirus) that affects trout, perches, mosquito fish and mountain galaxias. EHNV is a large icosahedral virus, approximately 175 nm with a double-stranded DNA genome of 127 kb [10]. The virus replicates in both the nucleus and cytoplasm with intracytoplasmic assembly. It obtains its outer limiting membrane via budding from the host cell plasma membrane. The inner capsid is surrounded by an internal lipid bilayer similar to that described for frog virus (FV3) [61] and contains a nucleoprotein core consisting of a genome that is circularly permuted and terminally redundant [10]. The disease has been reported only in Australia. The disease may cause variable range of mortality and infects juveniles and adults within a temperature range of 11–17 °C [1]. The infected fish show distended abdomen, darkened skin, petechial haemorrhages at the base of fins and haemorrhages in gills. Grossly, the infected fish show swollen kidney and spleen. EHNV causes multifocal necrosis of the spleen and renal hematopoietic tissue as well as the liver. Microscopically, basophilic intracytoplasmic inclusion bodies are found in infected cells. Besides, hyperplasia and multifocal necrosis of gill epithelial cells, necrosis of atrial trabeculae and gastrointestinal epithelial cells, focal pancreatic necrosis, necrotic circulating hematopoietic cells and degenerate vascular endothelial cells in many organs are commonly noticed [37, 49, 59]. Further, ulcerative dermatitis, swim bladder oedema and necrosis have been described in EHNV-infected rainbow trout [49].
EHNV is extremely resistant to drying, surviving for months in water, and persisting in frozen fish tissues and carcasses for at least a year [36, 58]. There is no vaccine available for the control of EHN and hence, bio-security, routine surveillance and good husbandry practices that reduce physiological stressors play vital role in preventing this disease.
Infectious Haematopoietic Necrosis
Infectious haematopoietic necrosis virus (IHNV) is a rhabdovirus under family Rhabdoviridae related to viral haemorrhagic septicaemia virus. The genome consists of single molecule of negative-sense ssRNA that encodes six proteins, nucleoprotein, phosphoprotein, matrix protein, glycoprotein, non-virion protein and polymerase [42]. IHNV infects salmon, trout, arctic char, pike, and perch species. Non-piscine carriers include leeches (Piscicola sp.), mayfly (Callibaetis sp.) and gill lice (Salminicola sp.) [1]. IHNV has been officially reported from Russia, China, Iran, Japan and Korea [1, 10]. Young fish are most susceptible to IHNV disease. The infected fish showed darkening of skin, haemorrhages on the abdominal skin and in the iris, bleeding at base of fins, exophthalmia, ascites, and in fry, a white discharge from the vent and skin haemorrhages. The disease may cause significant mortality in young trout and salmon.
Fish surviving of disease caused by IHNV may act as carriers of the virus. The transmission generally occurs via water as the virus is present in all the secretions and excretions of infected fish. Blood-sucking parasites and fish eating birds may transfer the virus to new areas [1].
Viral Haemorrhagic Septicaemia
Viral haemorrhagic septicaemia virus (VHSV) is caused by Rhabdovirus of the genus Novirhabdovirus. In Asia, marine fish and trout are the most susceptible species. This disease is officially reported from Japan, Korea and Iran. The infected fish show rapid and high mortality. In acute cases, the fish show darkening of body colour, exophthalmia, bleeding around eyes and base of the fines, skin ulceration and pale gill with pin point haemorrhages. Grossly, the infected fish shows ascites, pin point haemorrhages in the fatty tissue, intestine, liver, swim bladder and muscle [1].
Water temperatures within a range of 4–14 °C are favourable for the growth of virus. The virus sheds from the host and is then transmitted through the water. The mortality may range from 10 to 80 %. Younger fish seem to be more susceptible to the disease. The existence of strain differences makes the different fish species more or less susceptible to the infection [1].
Infectious Pancreatic Necrosis
Infectious pancreatic necrosis virus (IPNV), is a Birnavirus (family Birnaviridae) that infects both freshwater and marine finfish and shellfish. Aquabirnavirus is the largest and most diverse of the three genera within the family Birnaviridae—non-enveloped viruses with a bi-segmented, double-stranded RNA genome and 60 nm in diameter [10]. One segment (A) encodes a polyprotein which is post-translationally cleaved to form three viral proteins VP2, VP3 and VP4, with VP2 epitopes being responsible for serotype specificity and the target for neutralizing antibodies [17]. The other segment (B) encodes VP1, an RNA-dependent RNA polymerase [18]. The four serogroups evident so far, A, B, C, D [16, 31, 33] have been described, with most aquabirnaviruses comprising nine serotypes (A1–A9) within serogroup A. These serotypes appear to correlate with geographical regions rather than host species [17]. Viruses within the other three serogroups are less well studied. Genetic analysis indicates the clustering of seven genogroups [44, 63] that tend to correlate with geographical and serological characteristics [12]. IPNV was the first fish virus isolated in cell culture [60] and until recently has remained one of the most intensely studied viruses of fish. Atlantic salmon, trout and zebra fish are affected by IPNV. Among fresh water fish carps and gold fish are affected. This disease has been reported from Iran, Japan, Korea and Australia. The infected fish show mortality ranging from 10 to 90 %, mostly in early stage of life. Clinically, the infected fish show signs of spiral swimming, swollen abdomen, darkened skin, exophthalmia, lethargy, and whitish faecal casts. On necropsy, the infected fish show ulcerative lesions in pancreas, oesophagus and stomach along with empty or mucous filled intestine [1].
The IPN disease is highly contagious and the infection spreads via water. The virus enters fish through gill or by ingestion. The virus is also vertically transmitted via infected eggs. The virus sheds in faeces, urine, spawning fluid and mucous. Blood-sucking parasites and fish-eating birds also transmit the infection. The survivors of the infection act as carriers of the virus. The virus is non-host specific and more than 20 different fish families are known to be infected with this disease.
Koi Herpes Virus
The Koi herpes virus disease is caused by Cyprinid herpesvirus-3 (CyHV-3) in the family Alloherpesviridae [13]. Its genome consists of 295 kb dsDNA [3]. CyHV-3 can be differentiated from Cyprinid herpesvirus-1 (CyHV-1) which causes carp pox, and from haematopoietic necrosis herpesvirus of goldfish, Cyprinid herpesvirus-2 (CyHV-2), by clinical signs, host range, antigenic properties, growth characteristics, cytopathic effects (CPE) in cell culture, and by DNA sequence [28].
Common carp and koi carp of all ages are known to be most susceptible to this virus. Recently, CyHV-3 was also detected in gold fish (Carasssius auratus auratus) and crucian carp (Carassius carassius) [19, 29, 50]. Davidovich et al. [11] was able to propagate the virus in cells derived from common carp, koi, silver carp and gold fish, indicating the potential vulnerability of other cyprinids to this virus.
KHV emerged as a highly virulent disease in the late 1990s as a serious threat to the common carp industry. After the first report of this disease in Israel, it has been reported in US and many other Asian and European countries [27, 40, 45, 47, 53, 54]. This disease has been reported form Indonesia, Japan, Taiwan, Israel, Malaysia, Thailand, Singapore, Philippines, Hong Kong and Korea [1, 48]. The infected fish show signs of erratic swimming behaviour, disorientation, gasping at the surface and high mortality. On necropsy, severe gill necrosis (Fig. 1), pale gill and skin, haemorrhages on skin and gill are generally noticed. The experimentally infected koi carp showed interstitial and glomerulonephritis, periglomerular fibrosis, hyperplastic changes in renal tubules, loss of gill lamellae, hyperplasia of gill epithelial cells leading fusion of lamellae, hyperplastic changes in stomach and intestine [41].
Fig. 1.
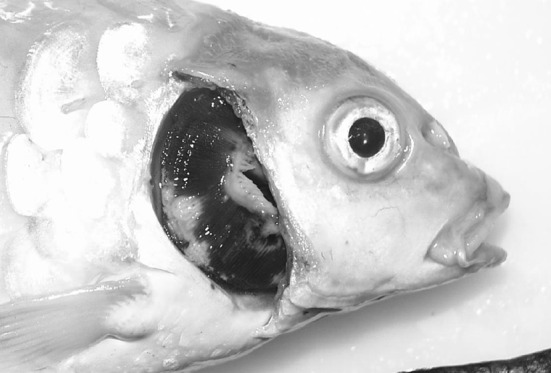
A koi (Cyprinus carpio) experimentally infected with CyHV-3 (koi herpesvirus). The pale areas seen on the gills are necrotic lesions typical of CyHV-3 disease. (Color figure online)
KHV epidemiology is greatly driven by environmental temperature. The virus survives at low temperature but disease outbreaks occur at 17–28 °C. No morbidities have been reported at 13 and 30 °C [48]. The disease progression is very fast in summer and slow in winter [46]. However, outbreaks are generally observed in spring or autumn and do not occur when water temperatures are high in summer or low in winter [22, 62].
CyHV-3 DNA has been detected in the droppings and gill and brain of infected fish [15], environmental water samples before, during or after outbreaks [24, 38]. Plankton, like rotifers, may also be associated with viral transmission [39].
Disruption of normal seasonal patterns in water temperature can be effective strategy for controlling this infection [46]. However, it has been reported that fish treated in this way may become symptomatic again after temperatures return to the permissive range [32] and it is likely that survivors serve as a source of future infections of naive fish. A vaccine for this virus is commercially available.
Infectious Spleen and Kidney Necrosis
Infectious spleen and kidney necrosis virus (ISKNV) is an iridovirus that infects invertebrates and poikilothermic vertebrates. The Iridoviridae are divided into five genera consisting of Iridovirus, Lymphocystivirus, Chloriridovirus, Ranavirus and Megalocytivirus. Of these genera, Megalocytivirus was identified as a new genus to the family [9]. ISKNV is selected as the type species of genus Megalocytivirus. The genome is 111 kb in length and contains 124 potential ORFs [26]. Fu et al. [20] constructed a phylogenetic tree with 33 megalocytiviruses and classified viruses into three genotypes and found a strong host species signal in three genotypes: for genotype I, the host was mainly marine fish; for genotype II, the host was freshwater fish; and for genotype III, the host was mainly flatfish.
ISKNV has been reported to cause infection in large number of fish species (50 species including the order Perciformes, Pleuronectiformes, Clupeiformes, Tetraodontiformes, Myctophiformes, Mugiliformes in China) with high mortality in both freshwater and marine species [56]. The virus infected fish tissue reveals hypertrophied cells in spleen, kidney, cranial connective tissue and endocardium with presence of cytoplasmic inclusions [57].
Viral Encephalopathy and Retinopathy (VER) Viral Nervous Necrosis (VNN)
VER/VNN is caused by a betanodavirus of the family Nodaviridae. The virus infect at least 40 species of fish in both freshwater and marine environments [43]. The disease was first reported in barramundi (Lates calcarifer) farmed in Australia [43] and subsequently in many parts of the world including India [10]. VNN is a small (25–30 nm in diameter), spherical, non-enveloped virus with positive sense ssRNA and the genome consists of two RNA molecules (RNA1 and RNA2). The RNA1 encodes for non-structural RNA-dependent RNA polymerase and RNA2 encodes the coat protein. At least five genotypes based on coat protein have been described from different geographical locations [21]. The typical histological picture of vacuolating necrosis of neural cells of the brain, retina and spinal cord aids in diagnosis of this disease. The virus may lead to 100 % mortality in larval and juvenile fish and the adults carry the virus. The survivors act as carrier of virus and vertical transmission of infection has been described.
Lymphocystis Disease
Lymphocystis disease is caused by an Iridovirus belonging to the genus Lymocystisvirus. This disease is a chronic disease and has been reported worldwide in a broad range of fresh water and marine water fish. The infected fish develop granular-nodules on the skin, fins, and sometimes the gills (Fig. 2). The growths may reach more than 2 cm in diameter and are composed of granules of 0.3–2.0 mm diameter that vary in colour. Each granule is a single epithelial cell distended by a crystalline accumulation of virus particles (Fig. 3). The nodules may take 1–3 months to develop. Lesions typically develop during cool seasons and then resolve when temperature increases in warmer months [5]. This infection is seldom fatal to fish.
Fig. 2.

A largemouth bass (Micropterus salmoides) infected by the lymphocystis virus. The large tumor-like growths on the pectoral and anal fins are composed of epithelial cells enlarged by accumulations of crystalline arrays of the lymphocystis iridovirus
Fig. 3.
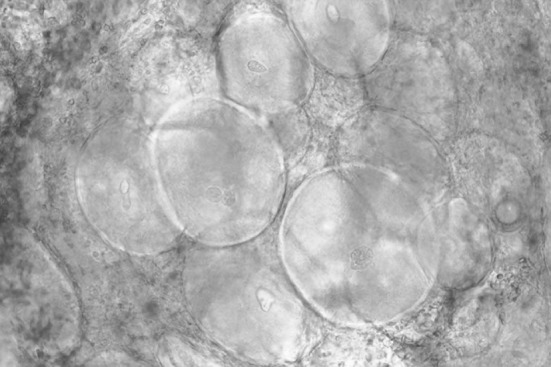
A wet mount of tissue from the fin of a largemouth bass (Micropterus salmoides) infected by the lymphocystis virus. The large round cells are epithelial cells containing accumulations of the lymphocystis iridovirus. The diameters of the larger cells are 300–500 μm
Carp Pox
Carp pox is caused by Cyprinid herpesvirus-1 (CyHV-1) and is a disease of common carp [51]. The disease has been reported in U.S.A, European countries, Russia, Malaysia, Japan, Israel and Korea. The affected fish develop milky-white or grey plaques, with a waxy or gelatinous appearance, on the body surface and fins (Fig. 4) [5]. The lesions are histologically-similar to papillomas but are better described as benign epidermal hyperplasia. The lesions typically develop during cool seasons and resolve when temperature increases and the fish immune system becomes more immunologically active. Mortality attributable to CyHV-1 infection is rare, but severe cases may lead to secondary infections or scarring.
Fig. 4.

A koi carp (Cyprinus carpio) with a CyHV-1 infection (carp pox). The pink plaques present on the head of the fish are focal proliferations of infected epithelial cells. (Color figure online)
Herpesviral Hematopoeitic Necrosis of Goldfish
Herpesviral Hematopoeitic Necrosis of Goldfish virus (HVHNV) produces serious epizootics among all ages of goldfish, Carrasius auratus, during the spring and fall seasons. Lesions associated with these outbreaks included pale gills, ascites, splenomegaly with white nodules, swollen kidney and anorexia. The virus responsible for HVHN is the herpesvirus Cyprinid herpesvirus-2 (CyHV-2) [35]. The disease has been reported in Western Japan [35], Taiwan [7], and in a private goldfish collection in Australia [52]. Surveys in North America show that CyHV-2 is widespread on commercial goldfish farms and outbreaks apparently occur when healthy carriers are subjected to a sharp temperature drop followed by holding at the permissive temperature (15–25 °C) for the disease [23]. There have been no organized surveys of the prevalence of CyHV-2 on Asian fish farms, but it is known to be present [7, 35, 52] and it exists at high prevalence in other regions known to be positive [23].
Chinese Grass Carp Reovirus (CGRV) Disease
The CGRV is responsible for an acute hemorrhagic disease in grass carp (Ctenopharyngodon idella) in China and is considered the most important disease of grass carp [4, 8]. Chinese grass carp reovirus also produces disease in other cyprinids including black carp (Mylopharyngodon piceus), stone moroko (Pseudorasabora parva) [14], and Chinese rare minnow (Gobiocypris rarus) in China [55], and has been detected in apparently healthy silver carp (Hypophthalmichtyhys molitrix) and Chinese minnow (Hemiculter bleekeri) [14, 34].
Conclusion
Among a lengthy list of viral pathogens of finfish, SVC, VEN, KHV and IPNV are the major causes of economic loss to the freshwater aquaculture sector of the Asia–Pacific region. Many countries seem to be devoid of any viral disease incidence in fish, including India, but this may be because the viral pathogens of regional fish species have not been studied in great detail. Even though viral diseases are being reported in many counties, there has been no systematic study conducted to estimate economic losses. However, the obvious economic importance of viral diseases in aquaculture justify greater efforts to strengthen the research, quarantine, and surveillance systems in the Asian–Pacific region.
References
- 1.AGDAFF-NACA. Aquatic animal diseases significant to Asia–Pacific: identification field guide. Australian Government Department of Agriculture, Fisheries and Forestry. Canberra; 2007.
- 2.Alderman DJ. Geographical spread of bacterial and fungal diseases of crustaceans. Rev Sci Technol Off Int Epiz. 1996;15:603–632. doi: 10.20506/rst.15.2.943. [DOI] [PubMed] [Google Scholar]
- 3.Aoki T, Hirono I, Kurukawa K, Fukuda H, Nahary R, Eldar A, Davison AJ, Waltzek TB, Bercovier H, Hedrick R. Genome sequences of three koi herpesvirus isolates representing the expanding distribution of an emerging disease threatening koi and common carp worldwide. J Virol. 2007;81:5058–5065. doi: 10.1128/JVI.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attoui H, Fang Q, Jaafar FM, Cantaloube JF, Biagini P, de Micco P, de Lamballerie X. Common evolutionary origin of aquareoviruse and orthoreoviruses revealed by genome characterization of Golden shiner reovirus, Grass carp reovirus, Striped bass reovirus and golden ide reovirus (genus Aquareovirus, family Reoviridae) J Gen Virol. 2002;83:1941–1951. doi: 10.1099/0022-1317-83-8-1941. [DOI] [PubMed] [Google Scholar]
- 5.Bernoth E-M, Crane MSJ. Viral diseases of aquarium fish. Semin Avian Exotic Pet Med. 1995;4:103–110. doi: 10.1016/S1055-937X(05)80046-5. [DOI] [Google Scholar]
- 6.Bondad-Reantaso MG, Subasinghe RP, Richard Arthur J, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M. Disease and health management in Asian aquaculture. Vet Parasitol. 2005;132:249–272. doi: 10.1016/j.vetpar.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Chang P-H, Lee S-H, Chiang H-C, Jong M-H. Epizootic of herpes-like virus infection in goldfish, Carassius auratus in Taiwan. Fish Pathol. 1999;34:209–210. doi: 10.3147/jsfp.34.209. [DOI] [Google Scholar]
- 8.Chen BS, Jiang Y. Morphological and physicochemical characterization of the hemorrhagic viruses of grass carp. Kexue Tonboga. 1984;29:832–835. [Google Scholar]
- 9.Chinchar VG, Essbauer S, He JG, Hyatt A, Miyazaki T, Seligy V, Williams T. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U and Ball LA (eds) Virus Taxonomy, VIIIth Report of the ICTV. Elsevier/Academic Press, London, 2005, pp. 145–162
- 10.Crane M, Hyatt A. Viruses of fish: an overview of significant pathogens. Viruses. 2011;3:2025–2046. doi: 10.3390/v3112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidovich M, Dishon A, Ilouze M, Kotler M. Susceptibility of cyprinid cultured cells to Cyprinid herpesvirus-3. Arch Virol. 2007;152:1541–1546. doi: 10.1007/s00705-007-0975-4. [DOI] [PubMed] [Google Scholar]
- 12.Davies KR, McColl KA, Wang L-F, Yu M, Williams LM, Crane MStJ. Molecular characterisation of Australasian isolates of aquabirnavirus. Dis Aquat Org. 2010;93:1–15. doi: 10.3354/dao02278. [DOI] [PubMed] [Google Scholar]
- 13.Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, Pellett PE, Roizman B, Studdert MJ, Thiry E. The order Herpesvirales. Arch Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Q, Yu L, Wang X, Ke L. Study on infecting other fishes with grass carp hemorrhagic virus. Chin J Virol. 1991;6:371–373. [Google Scholar]
- 15.Dishon A, Perelberg A, Bishara-Shieban J, Ilouze M, Davidovich M, Werker S, Kotler M. Detection of carp interstitial nephritis and gill necrosis virus in fish droppings. Appl Environ Microbiol. 2005;71:7285–7291. doi: 10.1128/AEM.71.11.7285-7291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon PF, Ngoh GH, Stone DM, Chang SF, Way K, Kueh SLF. Proposal for a fourth aquabirnavirus serogroup. Arch Virol. 2008;153:1937–1941. doi: 10.1007/s00705-008-0192-9. [DOI] [PubMed] [Google Scholar]
- 17.Dobos P. The molecular biology of infectious pancreatic necrosis virus (IPNV) Ann Rev Fish Dis. 1995;5:25–54. doi: 10.1016/0959-8030(95)00003-8. [DOI] [Google Scholar]
- 18.Dobos P, Hill BJ, Hallet R, Kells DT, Becht H, Teninges D. Biophysical and biochemical characterisation of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979;32:593–605. doi: 10.1128/jvi.32.2.593-605.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Matbouli M, Saleh M, Soliman H. Detection of Cyprinid herpesvirus-3 (CyHV-3) in goldfish (Carassius auratus) Vet Rec. 2007;161:792–793. [PubMed] [Google Scholar]
- 20.Fu X, Li N, Liu L, Lin Q, Wang F, Lai Y, Jiang H, Pan H, Shi C, Shuqin W. Genotype and host range analysis of infectious spleen and kidney necrosis virus. Virus Genes. 2011;42:97–109. doi: 10.1007/s11262-010-0552-x. [DOI] [PubMed] [Google Scholar]
- 21.Gagné N, Johnson SC, Cook-Versloot M, MacKinnon AM, Olivier G. Molecular detection and characterization of nodavirus in several marine fish species from the northeastern Atlantic. Dis Aquat Org. 2004;62:181–189. doi: 10.3354/dao062181. [DOI] [PubMed] [Google Scholar]
- 22.Gilad O, Yun S, Andree KB, Adkinson A, Zlotkin A, Bercovierm H, Elderm A, Hedrick RP. Molecular comparison of isolates of an emerging fish pathogen, koi herpes virus, and the effect of water temperature on mortality of experimentally infected koi. J Gen Virol. 2003;84:2661–2667. doi: 10.1099/vir.0.19323-0. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin AE, Sadler J, Merry GE, Marrcaux EN. Herpesviral haematopoietic necrosis virus (CyHV-2) infection: case studies from commercial goldfish farms. J Fish Dis. 2009;32:271–278. doi: 10.1111/j.1365-2761.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- 24.Haramoto E, Kitajima M, Katayama H, Ohgaki S. Detection of koi herpesvirus DNA in river water in Japan. J Fish Dis. 2007;30:59–61. doi: 10.1111/j.1365-2761.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 25.Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofman EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW, Smith GW, Vasta GR. Emerging marine diseases: climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 26.He JG, Deng M, Weng SP, Li Z, Zhou SY, Long QX, Wang XZ, Chan SM. Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology. 2001;291:126–139. doi: 10.1006/viro.2001.1208. [DOI] [PubMed] [Google Scholar]
- 27.Hedrick RP, Gilad O, Yun S, Spangenberg JV. A herpesvirus associated with mass mortality of juvenile and adult koi, a strain of a common carp. J Aquat Anim Health. 2000;12:44–57. doi: 10.1577/1548-8667(2000)012<0044:AHAWMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Hedrick RP, Gilad O, Yun SC, McDowell TS, Waltzek TB, Kelley GO, Adkison MA. Initial isolation and characterization of a herpes-like virus (KHV) from koi and common carp. Bull Fish Res Agency. 2005;2:1–7. [Google Scholar]
- 29.Hedrick RP, Waltzek TB, McDowell TS. Susceptibility of koi carp, common carp, goldfish and goldfish x common carp hybrids to cyprinid herpesvirus-2 and herpesvirus-3. J Aquat Anim Health. 2006;18:26–34. doi: 10.1577/H05-028.1. [DOI] [Google Scholar]
- 30.Hill B. National and international impacts of white spot disease of shrimp. Bull Eur Assoc Fish Pathol. 2002;22:58–65. [Google Scholar]
- 31.Hill BJ, Way K. Serological classification of infectious pancreatic necrosis (IPN) virus and other aquatic birnaviruses. Ann Rev Fish Dis. 1995;5:55–77. doi: 10.1016/0959-8030(95)00011-9. [DOI] [Google Scholar]
- 32.Iida T, Sano M. Koi herpesvirus disease. Uirusu. 2005;55:145–151. doi: 10.2222/jsv.55.145. [DOI] [PubMed] [Google Scholar]
- 33.John KR, Richards RH. Characteristics of a new birnavirus associated with a warm-water fish cell line. J Gen Virol. 1999;80:2061–2065. doi: 10.1099/0022-1317-80-8-2061. [DOI] [PubMed] [Google Scholar]
- 34.Jun L, Tiehui W, Yonglan Y, Hanquin L, Renhou L, Hongxi C. A detection method for grass carp hemorrhagic virus (GCHV) based on a reverse transcription polymerase chain reaction. Dis Aquat Org. 1997;29:7–12. doi: 10.3354/dao029007. [DOI] [Google Scholar]
- 35.Jung SJ, Miyazaki T. Herpesviral haematopoietic necrosis of goldfish, Carassius auratus (L.) J Fish Dis. 1995;18:211–220. doi: 10.1111/j.1365-2761.1995.tb00296.x. [DOI] [Google Scholar]
- 36.Langdon JS. Experimental transmission and pathogenicity of epizootic haematopoietic necrosis virus (EHNV) in redfin perch, Perca fluviatilis L., and 11 other teleosts. J Fish Dis. 1989;12:295–310. doi: 10.1111/j.1365-2761.1989.tb00318.x. [DOI] [Google Scholar]
- 37.Langdon JS, Humphrey JD, Williams LM. Outbreaks of an EHNV-like iridovirus in cultured rainbow trout, Salmo gairdneri Richardson, in Australia. J Fish Dis. 1988;11:93–96. doi: 10.1111/j.1365-2761.1988.tb00527.x. [DOI] [Google Scholar]
- 38.Minamoto T, Honjo MN, Uchii K, Yamanaka H, Suzuki AA, Kohmatsu Y, Iida T, Kawabata Z. Detection of cyprinid herpesvirus 3 DNA in river water during and after an outbreak. Vet Microbiol. 2009;135:261–266. doi: 10.1016/j.vetmic.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 39.Minamoto T, Honjo MN, Yamanaka H, Tanaka N, Itayama T, Kawabata Z. Detection of cyprinid herpesvirus-3 DNA in lake plankton. Res Vet Sci. 2011;90:530–532. doi: 10.1016/j.rvsc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki T, Okamoto H, Kageyama T, Kobayashi T. Viremia-associated Ana-Aqki-Byo, a new viral disease in color carp Cyprinus carpio in Japan. Dis Aquat Org. 2000;39:188–192. doi: 10.3354/dao039183. [DOI] [PubMed] [Google Scholar]
- 41.Mohi El-Din MM. Histopathological studies in experimentally infected koi carp (Cyprinus carpio koi) with koi herpesvirus in Japan. World J Fish Mar Sci. 2011;3:252–259. [Google Scholar]
- 42.Morzunov SP, Winton JR, Nichol ST. The complete genome structure and phylogenetic relationship of infectious hematopoietic necrosis virus. Virus Res. 1995;38:175–192. doi: 10.1016/0168-1702(95)00056-V. [DOI] [PubMed] [Google Scholar]
- 43.Munday BL, Kwang J, Moody N. Betanodavirus infections of teleost fish: a review. J Fish Dis. 2002;25:127–142. doi: 10.1046/j.1365-2761.2002.00350.x. [DOI] [Google Scholar]
- 44.Nishizawa T, Kinoshita S, Yoshimizu M. An approach for genogrouping of Japanese isolates of aquabirnaviruses in a new genogroup, VII, based on the VP2/NS junction region. J Gen Virol. 2005;86:1973–1978. doi: 10.1099/vir.0.80438-0. [DOI] [PubMed] [Google Scholar]
- 45.Oh MJ, Jung SJ, Choi TJ, Kim HR, Rajendran KV, Kim YJ, Park MA, Chun SK. A viral disease occurring in cultured carp Cyprinus carpio in Korea. Fish Pathol. 2001;36:147–151. doi: 10.3147/jsfp.36.147. [DOI] [Google Scholar]
- 46.Omori R, Adams B. Disrupting seasonality to control disease outbreaks: the case of koi herpes virus. J Theor Biol. 2011;271:159–165. doi: 10.1016/j.jtbi.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Perelberg A, Smirnov M, Hutoran M, Diamant A, Bejerano Y, Kotler M. Epidemiological description of a new viral disease afflicting cultured Cyprinus carprio in Israel. Israel J Aqua Bamidgeh. 2003;55:5–12. [Google Scholar]
- 48.Pokorova D, Vesely T, Piackova V, Reschova S, Hulova J. Current knowledge on koi herpesvirus (KHV): a review. Vet Med Czech. 2005;5:139–147. [Google Scholar]
- 49.Reddacliff LA, Whittington RJ. Pathology of epizootic haematopoietic necrosis virus (EHNV) infection in rainbow trout (Oncorhynchus mykiss Walbaum) and redfin perch (Perca fluviatilis L) J Comp Pathol. 1996;115:103–115. doi: 10.1016/S0021-9975(96)80033-8. [DOI] [PubMed] [Google Scholar]
- 50.Sadler J, Marecaux E, Goodwin AE. Detection of koi herpesvirus (CyHV-3) in goldfish, Carassius auratus (L.), exposed to infected koi. J Fish Dis. 2008;31:71–72. doi: 10.1111/j.1365-2761.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 51.Sano T, Fukuda H, Furukawa M. Herpesvirus cyprini: biological and oncogenic properties. Fish Pathol. 1985;20:381–388. doi: 10.3147/jsfp.20.381. [DOI] [Google Scholar]
- 52.Stephens FJ, Raidal SR, Jones B. Haematopoietic necrosis in a goldfish (Carassius auratus) associated with an agent morphologically similar to herpesvirus. Aust Vet J. 2004;82:167–169. doi: 10.1111/j.1751-0813.2004.tb12650.x. [DOI] [PubMed] [Google Scholar]
- 53.Taylor NGH, Dixon PF, Jeffery KR, Peeler EJ, Denham KL, Way K. Koi herpesvirus: distribution and prospects for control in England and Wales. J Fish Dis. 2009;33:221–230. doi: 10.1111/j.1365-2761.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- 54.Walster C. Clinical observations of severe mortalities in koi carp, Cyprinus carpio, with gill disease. Fish Vet J. 1999;3:54–58. [Google Scholar]
- 55.Wang T, Chen H, Liu H, Yi Y, Guo W. Preliminary studies of the susceptibility of Gobiocyprisrarus to hemorrhagic virus of grass carp. Acta Hydrobiol Sin. 1994;18:144–149. [Google Scholar]
- 56.Wang YQ, Lu L, Weng SP, Huang JN, Chan SM, He JG. Molecular epidemiology and phylogenetic analysis of a marine fish infectious spleen and kidney necrosis virus-like (ISKNV-like) virus. Arch Virol. 2007;152:763–773. doi: 10.1007/s00705-006-0870-4. [DOI] [PubMed] [Google Scholar]
- 57.Weng SP, He JG, Zeng K, Huang ZJ, Hou KT, Huang WP, Chen JH, Luo JR. Infectious spleen and kidney necrosis virus infection in Siniperca chuatsi: histopathology and relationship with HB, RBC and WBC. J South China Norm Univ. 1998;82:70–76. [Google Scholar]
- 58.Whittington RJ, Kearns C, Hyatt AD, Hengstberger S, Rutzou T. Spread of epizootic haematopoietic necrosis virus (EHNV) in redfin perch (Perca fluviatilis) in southern Australia. Aus Vet J. 1996;73:112–114. doi: 10.1111/j.1751-0813.1996.tb09992.x. [DOI] [PubMed] [Google Scholar]
- 59.Whittington RJ, Becker JA, Dennis MM. Iridovirus infections in finfish: a critical review with emphasis on ranaviruses. J Fish Dis. 2010;33:95–122. doi: 10.1111/j.1365-2761.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 60.Wolf K, Sniesko SF, Dunbar CE, Pyle E. Virus nature of infectious pancreatic necrosis in trout. Proc Soc Exp Med Biol. 1960;104:105–108. doi: 10.3181/00379727-104-25743. [DOI] [PubMed] [Google Scholar]
- 61.Yan X, Yu Z, Zhang P, Battisti AJ, Holdaway HA, Chipma PR, Bajaj C, Bergoin M, Rossmann MG, Baker TS. The capsid proteins of a large, icosahedral dsDNA virus. J Mol Biol. 2009;385:1287–1299. doi: 10.1016/j.jmb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuasa K, Ito T, Sano M. Effect of water temperature on mortality and virus shedding in carp experimentally infected caps with koi herpes virus. Fish Pathol. 2008;43:83–85. doi: 10.3147/jsfp.43.83. [DOI] [Google Scholar]
- 63.Zhang CX, Suzuki S. Aquabirnaviruses isolated from marine organisms form a distinct genogroup from other aquabirnaviruses. J Fish Dis. 2004;27:633–643. doi: 10.1111/j.1365-2761.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang NZ, Zhang LF, Jiang YN, Zhang T, Xia C. Molecular analysis of spring viraemia of carp virus in China: a fatal aquatic viral disease that might spread in east Asian. PLoS ONE. 2009;4:e6337. doi: 10.1371/journal.pone.0006337. [DOI] [PMC free article] [PubMed] [Google Scholar]


