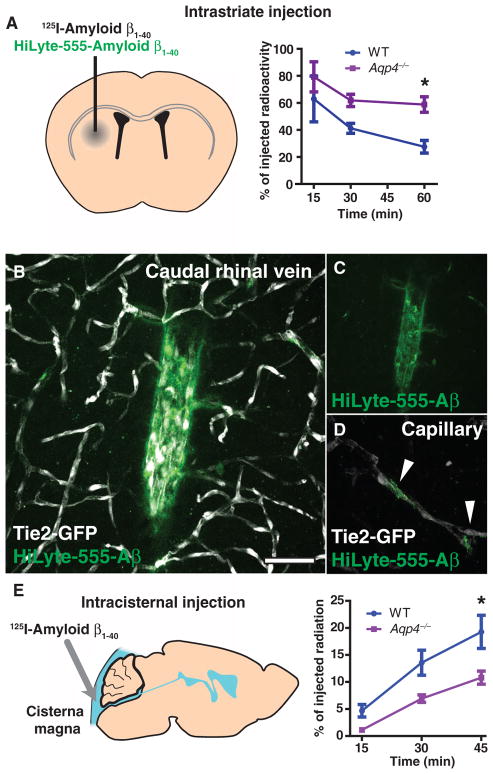
Fig. 6.
Interstitial Aβ is cleared along paravascular pathways. To evaluate whether interstitial soluble amyloid Aβ is cleared along the same pathways as other tracers, we injected fluorescent or radiolabeled amyloid β1–40 into the mouse striatum. (A) Fifteen minutes, 30 min, or 1 hour after 125I-amyloid β1–40 injection, whole-brain radiation was measured, as detailed in fig. S8. At t = 60 min in WT animals, 125I-amyloid β1–40 was cleared more rapidly than [3H]mannitol or [3H]dextran-10. 125I-Amyloid β1–40 clearance in Aqp4-null mice was significantly reduced (*P < 0.05, n = 4 to 6 per time point). (B to D) One hour after injection with HyLyte-555–amyloid β1–40 into Tie2-GFP mice, tracer accumulated along capillaries (D, arrows) and large draining veins (B and C). Image in (C) depicts (B) without the endothelial GFP fluorescence signal. (E) To evaluate whether soluble Aβ within the CSF could recycle through the brain parenchyma, we injected 125I-amyloid β1–40 intracisternally, and we evaluated radiotracer influx into the brain (as in fig. S2) 15, 30, and 45 min after injection. 125I-Amyloid β1–40 entered the brain in a manner comparable to [3H]dextran-10, and compared to WT controls, 125I-amyloid β1–40 influx was significantly reduced in Aqp4-null mice (*P < 0.05, n = 4 to 6 per time point). Scale bar, 50 μm.