Abstract
Objectives:
To study the results of magnetic resonance-guided focused ultrasound surgery (MRgFUS) treatment carried out on Indian patients in our Hospital.
Materials and Methods:
Fifty Indian women (mean age = 36.2 ± 8.3 years) were treated for fibroids as outpatients using the ExAblate MRgFUS system (InSightec). Non-perfused volumes (NPVs) were measured immediately after treatment to calculate the treatment outcomes. A validated symptom-specific questionnaire to record their symptoms prior to treatment and six months following treatment was completed by patients. The size of the fibroids was measured on the day of the treatment and during the 6-month checkup to calculate shrinkage. Adverse events during and following treatment were recorded and monitored.
Results:
The average NPV ratio measured after the treatment was 88% ± 6%, indicative of high ablated fibroid tissue. Prior to treatment, the mean Symptoms Severity Score was 56.9 ± 4.8 (n = 50), which is indicative of highly symptomatic patients. Six months following treatment, there was an average fibroid shrinkage of 30% ± 11%, and a significant decrease in the mean score to 28.6 ± 6.0 (n = 50) (P < 0.001). There were no reports of serious or unexpected adverse events at any point during treatment or during the follow-up period from any of the 50 women treated in the current study.
Conclusions:
The current results obtained after 6 months of treatment corroborated previous data on the safety and efficacy of MRgFUS for treating uterine fibroids. This is the first publication that provides such data for a large cohort of Indian women.
Keywords: Magnetic resonance-guided focused ultrasound, noninvasive, uterine fibroids
INTRODUCTION

Uterine fibroids (leiomyomas) are the most widespread reproductive tract tumors in women during their childbearing years. A variety of symptoms are associated with fibroids including pelvic pain, heavy and prolonged menstrual flow, abdominal pressure, urinary frequency, and infertility, thereby significantly affecting quality of life.[1–3] The standard for surgical treatment of fibroids is myomectomy or hysterectomy,[4] with less invasive treatments being hormonal therapy, uterine artery embolization (UAE), and magnetic resonance-guided focused ultrasound surgery (MRgFUS).[4–7] The benefits of these minimally invasive strategies are that they spare the uterus,[8] require minimal or no hospitalization, reduce complication rates, treatment costs, and recovery time.[9,10] However, myomectomy and UAE are preferable to hysterectomy as they minimize recovery time. The use of myomectomy can be limited by the location and size of the fibroid. While UAE is often associated with complications such as post-embolization syndrome.[11]
In 2004, the ExAblate 2000® MRgFUS system was approved by the US Food and Drug Association (FDA) for the treatment of uterine fibroid by thermal ablation.[12] MRgFUS is a non-invasive treatment that utilizes high intensity focused ultrasound to target multiple focal spots, under real-time guidance and monitoring using magnetic resonance imaging (MRI), thereby providing anatomical and thermal feedback. The temperature of the tissue within the focal zone is raised and ablation of the targeted tissue is achieved through protein denaturation, cell death, and coagulative necrosis.[7] For the past several years, MRgFUS has been in clinical use for the treatment of symptomatic fibroids.[7,12–16] For the treatment of uterine fibroids, MRgFUS has shown to be safe and efficacious, according to studies of several groups worldwide.[7,14–17] The duration of the procedure is approximately 3-4 hours.[13] A reduction in symptoms for a period of up to 2 years following treatment without any major complications has been reported.[7] Advantages of this procedure are that it can be performed as an outpatient treatment and only targeted areas are ablated, leaving surrounding tissue intact.
This article is the first to report the use of MRgFUS treatment for uterine fibroids in a large cohort of patients in India, with a 6-month follow-up evaluation. We describe here our initial experience with the MRgFUS system and report on the safety and short-term efficacy of the treatment on this specific patient population.
MATERIALS AND METHODS
Between July 2010 and December 2010, 50 women underwent uterine fibroid treatment in Jaslok Hospital and Research Center using the ExAblate 2000® MRgFUS system (InSightec Ltd., Tirat Carmel, Israel). Necessary approval from Ethics committee from Jaslok Hospital and Research Center was obtained before starting the study. To ensure patient safety and enhance efficacy, women who had bowel segment in the path of the ultrasound beam, between the transducer and the targeted fibroids, were excluded from the current study. We screened all the patients with pelvic MRI scan with contrast. The following inclusion and exclusion criteria were used for selection of the patients.
Inclusion criteria
Fibroids appearing hypointense and isointense on T2-weighted images and homogeneously enhancing on post-contrast study
Fibroids having a maximum diameter of 12 cm
Pedunculated fibroids with more than 50% attachment
Exclusion criteria
T2 hyperintense and post-contrast non-enhancing fibroids
Pedunculated fibroids with less than 50% attachment
Uterine posterior wall fibroids
Patients with contraindications to MRI such as patients with pacemakers, metallic implants, patients who were claustrophobic, and others
All patients were treated on the ExAblate 2000® MRgFUS system, which is integrated with a 1.5-T MRI scanner (GE Healthcare, Milwaukee, WI, USA) to produce real-time thermal images throughout the treatment stage of the procedure. Prior to initiation of treatment, patients’ abdomen was shaved and cleaned, a urinary catheter was inserted, and conscious sedation (Inj. Fentanyl [100 μg] + Midazolam [2 mg]) was administered. Each patient lay prone on the ExAblate treatment table with their abdomen positioned over the ultrasound transducer bath and a “stop sonication” button in their hands, which when pressed would immediately stop delivery of focused ultrasound energy.
Prior to initiation of treatment, a series of T2-weighted MR images in three orientations were acquired for identification of patients’ anatomy and procedure planning [Figure 1a]. The treatment boundaries and sensitive areas such as nerves, bowels, and bone were marked using the ExAblate software. Manual optimization of the sonication regions was performed by the attending physician using the pretreatment images and associated ExAblate software. During the treatment, a series of multiple sonications were applied over the course of approximately 3-4 h with a short cooling interval between each sonication. Immediately following the procedure, T1-weighted contrast-enhanced images were acquired in order to calculate the non-perfused volume (NPV) [Figure 1b]. Patients were given a dose of intravenous antibiotics (Injection Augmentin 1.2 g) kept overnight in the hospital before being discharged the next morning. This also ensured the treating doctor examined the patients before they left the hospital. Most of the patients returned to their normal daily activities including working regular hours within 1-2 days after treatment. Six months after the treatment, patients returned for a follow-up. MRI was used to measure the size of the fibroids and calculate the shrinkage [Figure 1c].
Figure 1a.
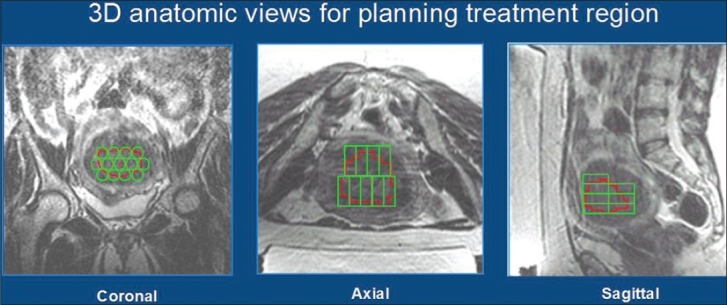
Axial, sagittal, and coronal T2-weighted magnetic resonance (MRI) images of the pelvic region to plan treatment of the fibroids
Figure 1b.
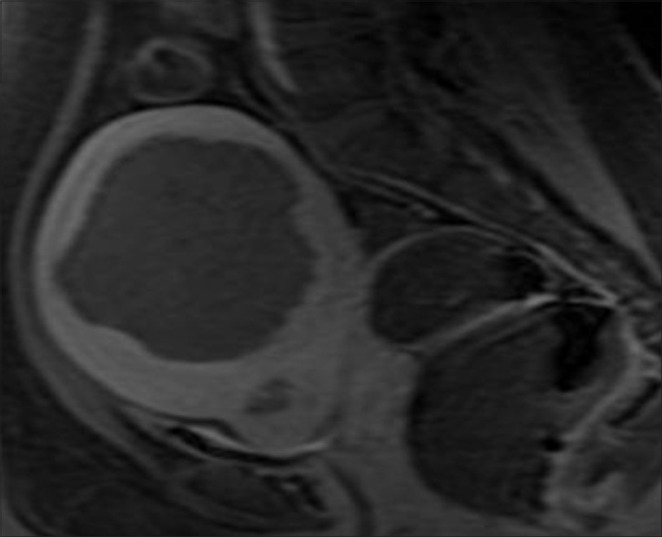
Post-treatment post-contrast T1-weighted magnetic resonance (MRI) images of the pelvic region shows the ablated fibroid (Approximate Fibroid volume 271 cc
Figure 1c.
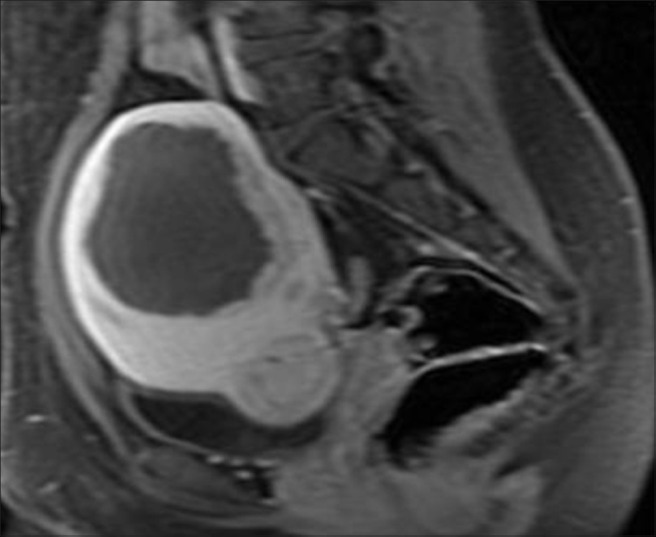
Six-month follow-up post-treatment post-contrast T1-weighted image shows 45-50% reduction in size of ablated fibroid (approximate volume 140 cc)
The uterine fibroid symptom and quality of life (UFSQOL) questionnaire was used to obtain an objective measure of the symptom level of the patients treated. The UFSQOL is a validated symptom-specific questionnaire, with questions targeting classical uterine fibroid symptoms, such as frequent urination, pressure, and menorrhagia, which is used to calculate a symptom severity score (SSS).[18] The most appropriate answer from a five-point scale is chosen by the patient and the final result is represented on a 100-point scale, with a lower score indicative of a lower severity of symptoms. The UFSQOL questionnaire was administered prior to treatment and 6 months following treatment.
The adverse events were recorded and monitored until they were resolved. Localized and transient pain in the abdominal region, fever, vaginal discharge, leg and lower back pain, and localized skin burns were defined as non-significant, anticipated adverse events.[19] Statistical analysis was performed, using the Student's Paired 2-sample t-test for mean value, to compare the mean SSS values between pretreatment and 6-month follow-up. Statistical significance was determined by a P value below 0.05.
RESULTS
The mean Body Mass Index (BMI) and age of the 50 Indian women treated with MRgFUS in the current study were 25.2 ± 1.6 (range: 21 ~ 28 Kg/m2) and 36.2 ± 8.3 years (mean ± SD; range: 21-53), respectively. The total mean fibroid volume was 119.5 ± 76.0 cc (range: 4.7 ~ 411.1 cc). All treated patients were premenopausal. Sixty-eight percent of the patients had a single fibroid, 26% of the patient had between two and four fibroids, and 6% had more than five fibroids. The mean number of treated fibroids per patient was 1.2 ± 0.5. Twenty patients (40%) had a second treatment and one patient (2%) had three treatments. An average of 84 sonications was performed per treatment, with average treatment duration (from first to last sonications) was 2:42 ± 0:48 h (n = 71 treatments).
Non-enhancing regions on T1-weighted contrast-enhanced images acquired immediately following the procedure showed an average NPV of 88% ± 6% of the fibroid volume. At 6-month follow-up, fibroid shrinkage was measured at 30% ± 11% decrease in fibroid volume (n = 61 fibroids). Prior to treatment, the mean SSS was 56.9 ± 4.8 (n = 50), which is indicative of highly symptomatic patients. Six months following treatment, there was a significant decrease in the mean SSS score to 28.6 ± 6.0 (n = 50) (P < 0.001).
There was no report of severe or unexpected adverse events from any of the 50 patients at any point during treatment or during the follow-up period. Fourteen women (28%) experienced adverse events. Immediately following the procedure, 10 incidences of leg pain were reported (20% of patients), nine of them resolved in 6 months after treatment, and one patient reported mild leg pain at the 6-month follow-up. In addition, one incidence each of urinary tract infection (2%), urine retention (2%), skin blister (2%), and abdominal pain (2%) were recorded following treatment, all resolved in 6 months after treatment.
DISCUSSION
The quality of life for women with uterine fibroids is severely impacted due to associated symptoms such as pelvic pain, abdominal pressure, urinary frequency, menstrual abnormalities, and even infertility. The available treatment options for uterine fibroids include invasive procedures such as hysterectomy and less invasive therapies such as UAE, myomectomy, myolysis, and most recently MRgFUS.[20] Patients with symptomatic uterine fibroids who wish to preserve their fertility and reduce their recovery time prefer such noninvasive therapies.
The advantages of MRgFUS treatment over other minimally invasive ones are that it is entirely non-invasive, can be performed as an outpatient treatment, has a low incidence of complications, and can be repeated, if necessary. MRgFUS is favorable, compared to other thermal ablative techniques, in that it is a noninvasive technique in which the focused ultrasound energy penetrates the body, but is absorbed significantly only when the beam reaches the focal spot. As a result, only the targeted regions are heated to coagulation level, thereby minimizing or eliminating potential damage to nearby tissues or organs. Since its approval by the FDA in 2004, MRgFUS has been shown in several studies to be a feasible and safe nonsurgical alternative for fibroid therapy.[14,21–23]
The current report provides corroborative safety and short-term clinical efficacy data on MRgFUS treatment for symptomatic uterine fibroids. It is the first report, however, to provide such data for a large cohort of Indian women. The 6-month follow-up data showed a significant reduction in the SSS and a large average NPV ratio, indicative of successful fibroid ablation. There were no major adverse events reported, and only one incidence of moderate leg pain, 6 months after treatment, was reported.
It is important to note that the UFSQOL questionnaire is widely used as an objective tool to assess treatment efficacy especially in minimally invasive uterine fibroid therapies, such as UAE and other noninvasive uterine fibroid therapies.[24,19] This assessment technique was developed to suit an ethnically diverse population of individuals and has since been implemented in a range of studies with cohorts of patients from various ethnic backgrounds.[7,17,24,25] Given that the current treatments were performed on a patient population of an ethnic background never before studied (Indian women), it is of significance that these results corroborate those of previous findings, particularly with those of US clinical trials where patient cohorts were of differing ethnic background.[19,14]
Although the study represents a short-term (6-month) analysis, the initial symptomatic relief described here is sufficient to conclude that MRgFUS can be effectively implemented as a treatment for women presenting with symptomatic uterine fibroids. The unique advantages of this treatment are that it is completely non-invasive, it can be performed on an outpatient basis, the recovery time is short, and the complication rate is low. Additional studies should be performed to determine the long-term safety and efficacy profiles of MRgFUS for the treatment of uterine fibroids in this and other population.
CONCLUSION
MRgFUS is a good and completely non-invasive treatment option for young patients. As per the data, the symptoms score improves faster with size reduction. This can be of great advantage to patients with significant SSSs at the beginning of treatment. Also, the postoperative recovery being fast, patients can get back to their regular activities almost the next day.
ACKNOWLEDGMENTS
The authors would like to thank Dr. G. Mansukhani, Dr. R. Pai, and Dr. I. Hinduja, Consultant Gynecologists, Jaslok Hospital and Research Center, Mumbai, India for their contributions to this study.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/74/104307
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vollenhoven BJ, Lawrence AS, Healy DL. Uterine fibroids: A clinical review. Br J Obstet Gynaecol. 1990;97:285–98. doi: 10.1111/j.1471-0528.1990.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 2.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–8. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 3.Middendorf K, Burges A, Strauss A, Hepp H. Uterine fibroids-therapy from the point of view of the gynaecologist. Radiologe. 2003;43:615–23. doi: 10.1007/s00117-003-0936-1. [DOI] [PubMed] [Google Scholar]
- 4.Lund N, Justesen P, Elle B, Thomsen SG, Floridon C. Fibroids treated by uterine artery embolization. A review. Acta Obstet Gynecol Scand. 2000;79:905–10. [PubMed] [Google Scholar]
- 5.Zupi E, Pocek M, Dauri M, Marconi D, Sbracia M, Piccione E, et al. Selective uterine artery embolization in the management of uterine myomas. Fertil Steril. 2003;79:107–11. doi: 10.1016/s0015-0282(02)04399-6. [DOI] [PubMed] [Google Scholar]
- 6.Levy BS. Modern management of uterine fibroids. Acta Obstet Gynecol Scand. 2008;87:812–23. doi: 10.1080/00016340802146912. [DOI] [PubMed] [Google Scholar]
- 7.Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–87. doi: 10.1097/01.AOG.0000275283.39475.f6. [DOI] [PubMed] [Google Scholar]
- 8.Andrews RT, Spies JB, Sacks D, Worthington-Kirsch RL, Niedzwiecki GA, Marx MV, et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2004;15:115–20. doi: 10.1097/01.rvi.0000109408.52762.35. [DOI] [PubMed] [Google Scholar]
- 9.Zowall H, Cairns JA, Brewer C, Lamping DL, Gedroyc WM, Regan L. Cost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroids. BJOG. 2008;115:653–62. doi: 10.1111/j.1471-0528.2007.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beinfeld MT, Bosch JL, Isaacson KB, Gazelle GS. Cost-effectiveness of uterine artery embolization and hysterectomy for uterine fibroids. Radiology. 2004;230:207–13. doi: 10.1148/radiol.2301021482. [DOI] [PubMed] [Google Scholar]
- 11.Lumsden MA. Embolization versus myomectomy versus hysterectomy: Which is best, when? Hum Reprod. 2002;17:253–9. doi: 10.1093/humrep/17.2.253. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. 2004. [Last accessed 2004 Oct 22]. Available from: http://www.fda.gov/cdrh/pdf4/p040003.html .
- 13.Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: Early results. AJR Am J Roentgenol. 2004;183:1713–9. doi: 10.2214/ajr.183.6.01831713. [DOI] [PubMed] [Google Scholar]
- 14.Fennessy FM, Tempany CM, McDannold NJ, So MJ, Hesley G, Gostout B, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery – results of different treatment protocols. Radiology. 2007;243:885–93. doi: 10.1148/radiol.2433060267. [DOI] [PubMed] [Google Scholar]
- 15.Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: Relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids-early experience. Eur J Obstet Gynecol Reprod Biol. 2008;139:199–203. doi: 10.1016/j.ejogrb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SW, Kim KA, Hwang YY. Magnetic resonance imaging-guided focused ultrasound surgery for uterine fibroids: Initial experience in Korea. Korean J Obstet Gynecol. 2008:51–70. [Google Scholar]
- 18.Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300. doi: 10.1016/s0029-7844(01)01702-1. [DOI] [PubMed] [Google Scholar]
- 19.Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–9. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 20.Munro MG. The evolution of uterine surgery. Clin Obstet Gynecol. 2006;49:713–21. doi: 10.1097/01.grf.0000211945.51712.99. [DOI] [PubMed] [Google Scholar]
- 21.Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417–30. doi: 10.1146/annurev.med.60.041707.170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, et al. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 23.Hudson SB, Stewart EA. Magnetic resonance-guided focused ultrasound surgery. Clin Obstet Gynecol. 2008;51:159–66. doi: 10.1097/GRF.0b013e318161e91f. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin SC, Spies JB, Worthington-Kirsch R, Peterson E, Pron G, Li S, et al. Uterine artery embolization for treatment of leiomyomata: Long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111:22–33. doi: 10.1097/01.AOG.0000296526.71749.c9. [DOI] [PubMed] [Google Scholar]
- 25.Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Hum Reprod Update. 2000;6:609–13. doi: 10.1093/humupd/6.6.609. [DOI] [PubMed] [Google Scholar]


