Abstract
Background:
To present the accumulated experience from treating chronic subdural hematomas (CSDH) in a local hospital of a third world country.
Methods:
One hundred and twenty-five consecutive patients with CSDH who were surgically treated in the Neurosurgical Department of the Hospital da Restauração, Recife-PE, Brazil, between January 2006 and May 2008, were retrospectively studied. Glasgow Outcome Scale (GOS) was employed to define outcome at 6 months as good (GOS 4 and 5) or poor (GOS ≤ 3). Age, admission Glasgow Coma Scale (GCS), location of hematomas (unilateral/bilateral), drainage system placement and recurrence were all analyzed for potential impact on final outcome.
Results:
The median age was 69 years, with a male/female ratio of 102/23. History of trauma was present in 60.8% of the patients. The median GCS on admission was 14. In 64 patients, the hematoma was on the left side, while in 42 patients it was on the right side. Bilateral hematomas were present in 19 cases (15.2%). Drainage systems were used in 93.6% of the cases. Recurrence occurred in 8.8% of the patients. One hundred and three patients obtained a good outcome at 6 months. The mortality rate was 11.2%. Patients with GCS ≥9 on admission presented better outcome (P < 0.05). Recurrent cases presented a poor outcome (P < 0.05).
Conclusions:
This study suggests that the main factors associated with outcome in patients harboring CSDH are the admission GCS score and the recurrence status. Advanced age is not a contraindication for surgical treatment. This study, solely focused on the Brazilian population, is the first of its kind in the English literature, and it could serve as a useful introduction to a more complex, multivariate, debate.
Keywords: Chronic subdural hematoma, outcome, recurrence, surgical treatment
INTRODUCTION
Chronic subdural hematomas (CSDHs) are defined as dark-red liquefied blood collections surrounded by a thin capsule, typically requiring about 3 weeks to develop.[20] They are commonly associated with old alcohol-addicted male patients, with a history of head trauma and concomitant coagulopathy.[1,4,10,12,28,32,33,35] The mortality rate varies between 8% and 15.6%.[4,7,28] However, specific groups of patients, such as those with coagulation abnormalities and older people, demonstrate a more elevated mortality rate.[4,35] In the past, several authors have analyzed the outcome of surgically treated patients with CSDH regarding factors such as bilateral localization, recurrence, placement or not of drainage systems, drainage blood volume, gender, admission Glasgow Coma Scale (GCS) and surgical technique, but the results were not always consistent.[4,7,10,11,18,19,23,24,28,30,32,35,36]
The authors present herein the outcome at 6 months of a series of 125 patients surgically treated for CSDH and explore the significance of various demographic, clinical and surgical variables. This is the first report (in the English literature) addressing this issue exclusively in Brazilian patients.
MATERIALS AND METHODS
During the period January 2006 to May 2008, 125 consecutive patients admitted to the Department of Neurosurgery (Hospital da Restauração, Recife-PE, Brazil), who received surgical treatment for CSDH, were retrospectively reviewed. Approval of the Research Ethic Committee of the Hospital was obtained. Data were retrieved from the patients’ medical records.
Diagnosis of CSDH was confirmed by computed tomography (CT) in all patients. General anesthesia was administered during operation. No operation in this series was performed with sedation or local anesthesia.
All patients underwent surgical intervention, which included burr hole and irrigation of the hematoma with saline solution. Most of the patients (n = 117, 93.6%) received one or two drainage systems for a time period of 3 days. If craniotomy was needed (recurrent cases), the capsule was removed as far as possible.
Outcome was evaluated by the Glasgow Outcome Scale (GOS) at 6 months, and classified patients in two groups; those with poor (GOS 1-3) and those with good outcome (GOS 4-5). The variables considered included age, admission GCS, unilateral or bilateral localization and recurrence. The relation between recurrence and drainage system insertion was also analyzed.
Statistical analyses were conducted using commercially available software (GraphPad Prism 4.0; GraphPad Software Inc: La Jolla, CA, USA). For 2 × 2 tables, the Chi-square test and the Fischer exact test (when one or more of the expected frequencies was <5) were performed for all variables. The level of significance was set at 5%.
RESULTS
Demographics-clinical presentation
The median age of the patients was 69 years (range: 5-98 years). The patients were divided in only two age groups due to their limited number; those being younger than 60 years and those older than 60 years. The male sex was dominant in this series, with a male/female ratio of 102/23.
The median value of the GCS at admission was 14 (range: 4-15). History of trauma was present in 76 patients (60.8%). Headache was a common symptom, occurring in 50 patients (40%), while contralateral motor deficit was present in 55 patients (44%). The basic features of the patients are provided in Table 1, including the underlying risk factors (i.e. anticoagulants, antiplatelet drugs, hypertension, etc.). Table 2 presents the major findings of similar articles found in the literature, none of which concern Brazilian patients.
Table 1.
Demographic and clinical features in 125 cases of CSDH
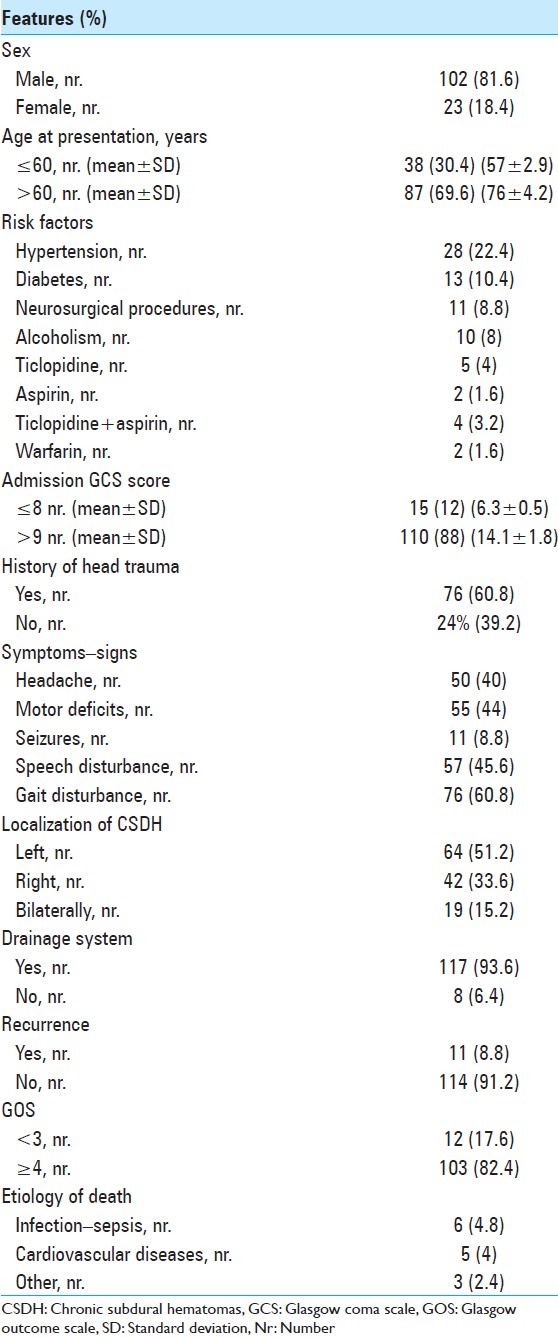
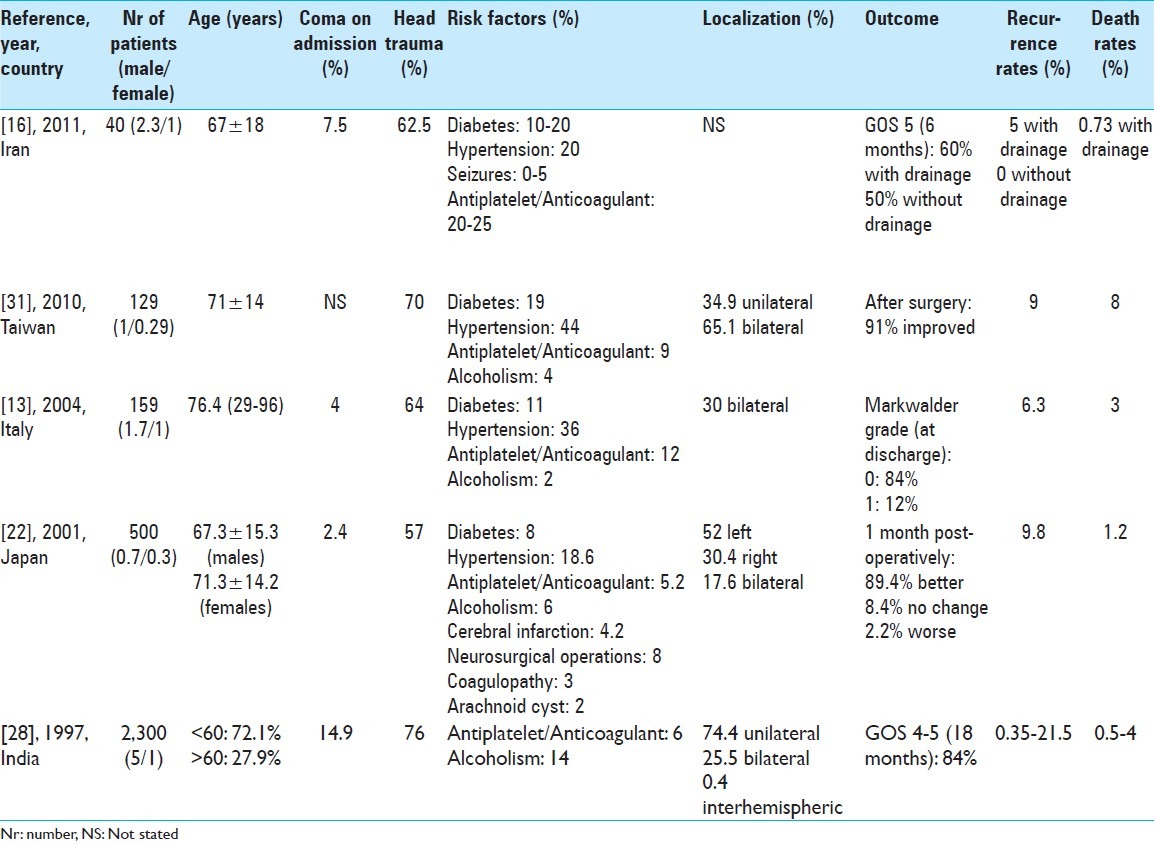
Table 2.
Indicative international studies on CSDH
Operative considerations
The hematoma was on the left side in 64 patients (51.2%) and on the right side in 42 patients (33.6%). Bilateral hematomas were present in 19 cases (15.2%). Sometimes, septa divided the subdural space in multiple compartments. Drainage systems were used in 117 patients (93.6%). Recurrence occurred in 11 patients (8.8%). There were no cases of postoperative meningitis. Wound infection occurred in three cases (2.4%).
Outcome
The median GOS at 6 months was 4 (range: 1-5). One hundred and three patients obtained a good outcome, representing 82.4% of the patients. Mortality rate was 11.2% (14 patients). In this series, six patients died of pulmonary infection or sepsis, five due to cardiovascular diseases and three due to unknown causes (recurrent hematomas).
Age was not statistically associated with outcome (P > 0.05) [Figure 1]. Bilateral hematomas were neither associated with outcome nor recurrence (P > 0.05) [Figures 2 and 3]. The placement or absence of placement of a drainage system did not yield statistically significant results with respect to recurrence (P > 0.05) [Figure 4].
Figure 1.
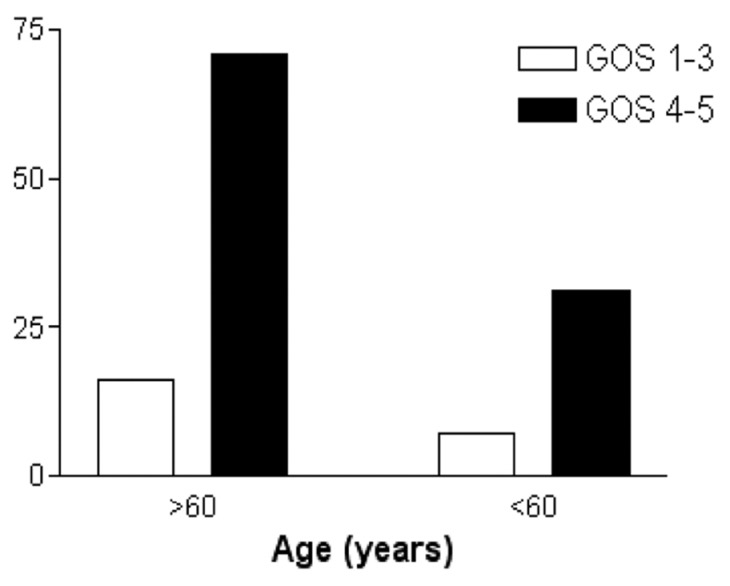
Age groups of patients according to outcome (Glasgow Outcome Score)
Figure 2.
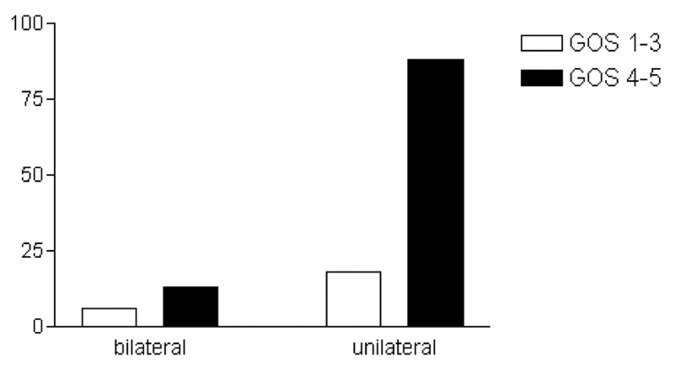
Location of chronic subdural hematoma and outcome (Glasgow Outcome Score)
Figure 3.
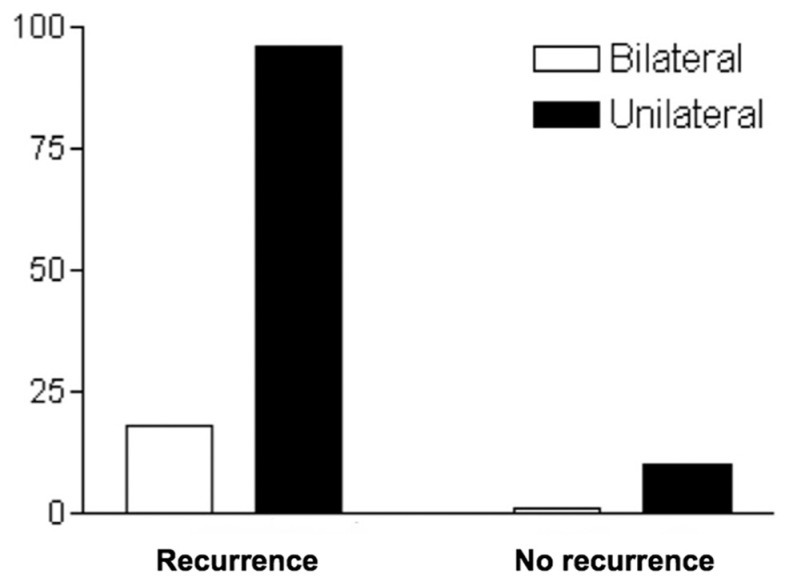
Recurrence of chronic subdural hematoma and location
Figure 4.
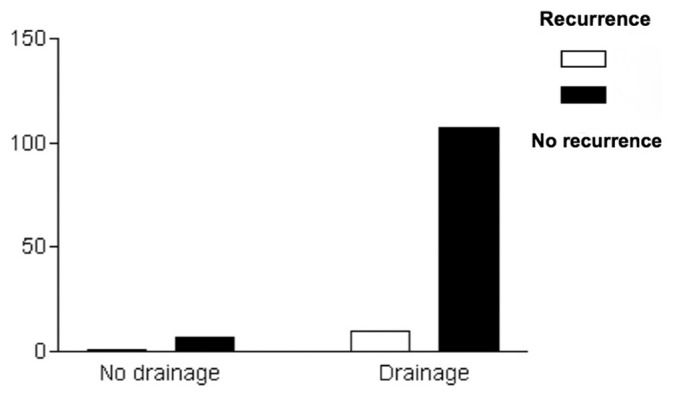
Use of drainage and recurrence
Patients presenting with an admission GCS score ≥9 had a statistically significant better outcome (P = 0.0033) [Figure 5]. As expected, patients with recurrent hematomas had poor outcome (P = 0.0066) [Figure 6]. Postoperative hydrocephalus and cerebrospinal fluid leak were not found to correlate with outcome in this study (P > 0.05).
Figure 5.
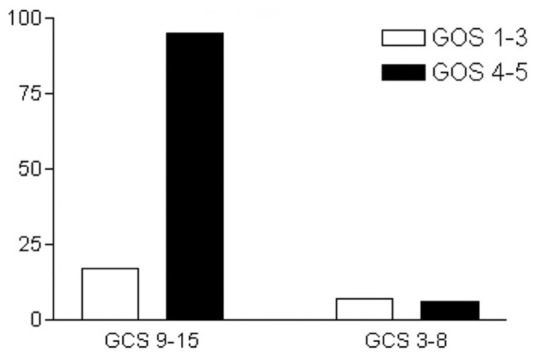
Admission Glasgow Come Scale and outcome (Glasgow Outcome Score)
Figure 6.
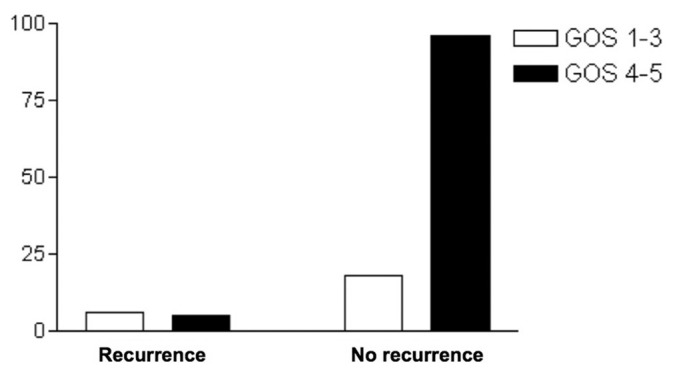
Recurrence of chronic subdural hematoma and outcome (Glasgow Outcome Score)
DISCUSSION
CSDH is a common surgical entity in the neurosurgical practice, but it still remains to be clarified which is the main factor influencing outcome [Table 2].
Demographics
CSDH is a condition mostly present in older people (50-70 years of age).[10,18,23,24] Men are more commonly affected than women.[10,24,27,35] Our patients had a history of head trauma in 60.8% of the cases, a finding consistent with the literature, which reports such a history varying between 35% and 75% of patients.[9,11,21,25,27,35] Furthermore, coagulation abnormalities represent a very important clinical factor associated with the presence, natural history and mortality rate of CSDH. A study has shown a higher mortality rate in the postoperative period in patients with international normalized ratio (INR) greater than 1.25 and/or thrombocytopenia.[35] Our data failed to assess this association because not all of our patients had available preoperative coagulation studies.
Surgical technique
In concern to the surgical technique, it is not clear which is the best surgical procedure for each patient. Some authors advocate twist drill craniostomy, others recommend burr hole surgery or even craniotomy. Most of them use drainage systems. Carmel et al. reported on a large series of patients operated by twist-drill craniostomy, where 86% of the patients achieved an excellent outcome.[7] However, the majority of the published literature agrees that optimal surgical treatment is achieved with the burr-hole trepanation surgery with a closed drainage system.[10,17,20,23,32,36] The outcome results based on those series are favorable in around 80% of the patients, similar to the results from the twist-drill craniostomy series.
Craniotomy still remains the principal treatment for some authors. Sambasivan in a very large series of 2300 cases of CSDH favors a small temporal craniotomy, with the dura left open and the subdural space communicating with the subtemporalis area.[28] Hamilton et al. compared 49 cases who underwent craniotomy and 43 who underwent burr-hole surgery, and found no significant differences between the two groups in relation to good outcome, postoperative complications, recurrence or mortality.[15]
Our data did not compare different surgical techniques because it is our protocol to routinely perform the burr-hole surgery as the first treatment modality. We retain the craniotomy option only for recurrent cases. The good outcome in 82.4% of our patients is comparable with other reports.
Recurrence
Recurrence has always been a source of frustration for neurosurgeons. Thus, it comes as no surprise that the vast majority of the published articles focus on this matter. According to the literature, the age of the patient, the history of seizure or diabetes mellitus, the bilateral localization, the architecture or width of the hematoma and the absence of placement of a drainage system are among the main reasons for failure.[2,4,20]
Most of the studies discouraging the use of a drainage system come from the beginning of the 1980s. Markwalder et al. reported a small series of 21 patients with CSDH in whom no drainage system was placed, and found no difference in the recurrence rate as compared with cases where closed drainage systems had been installed.[19] Robinson, in a large series of 133 cases treated with burr-hole surgery without drainage system, suggested that high-risks patients for recurrence are the elderly ones with bilateral hematomas and most patients in critical condition.[27] However, these studies are not comparative.
Wakai et al. conducted a prospective comparative study with 38 patients on the recurrence rate of CSDH after surgery with (group A) and without (group B) closed system drainage.[32] There were no significant differences between the two groups for sex, age, preoperative hematoma volume and density on CT scan. Recurrence rate in group A was 5% (one case) as opposed to 33% in group B (six cases) (P < 0.05). The authors conclude that closed system drainage reduces the recurrence rate of CSDH.
Yamamoto et al. analyzed independent predictors of recurrence of CSDH and found four variables associated with recurrence: presence of hematoma cavities on CT scans, history of seizure, width of the hematoma and history of diabetes mellitus.[34] Another report by Nakaguchi et al. emphasizes that the internal architecture of the hematoma is an important factor and that the recurrence rate is higher for separated types and cranial base hematomas.[24] Bilateral hematomas are also associated with a high risk of recurrence in the published literature.[20,29,30]
The direction of the drainage catheter during its placement has been also studied by some authors. Nakaguchi et al. believe that the incidence of postoperative fluid reaccumulation is reduced by placing the tip of the drainage catheter in the subdural frontal convexity and by removing subdural air during or after surgery.[23] Nevertheless, positioning the tip of the catheter in contact with the hematoma capsule in the subdural space may increase the risk of postoperative seizures and secondary intracranial infections.[36]
There seems to be a controversy regarding the postoperative drainage volume of CSDH. Kwon et al., reporting on a series of 175 patients with CSDH, stated that less postoperative drainage volume than expected is associated with a high possibility of recurrence.[18] The opposite is supported by Matsumoto et al., who published a series of 121 cases and concluded that the drainage volume was significantly larger in recurrent cases than in nonrecurrent cases.[20] The recurrence rate increased in proportion to the drainage volume. Other factors associated with recurrence were hematoma thickness and diabetes mellitus.[20]
Abouzari et al. analyzed another important factor for recurrence of CSDH, the role of postoperative patient posture.[1] Their conclusion was that assuming an upright posture soon after burr-hole surgery was associated with a significantly increased incidence of recurrence but not with a significant change in other position-related postsurgical complications.
Our recurrence rate was 8.8% in this series, a percentage similar to the ones previously published. Neither bilateral localization (P > 0.05) nor the use of drainage system (P > 0.05) was associated with recurrence according to these data. A drainage system was placed in almost 94% of our cases as a standard protocol procedure; therefore, the result might be biased. Thus, comparison between the recurrence rate in those who harbored a drainage system and those who did not cannot draw any statistically significant conclusions. From the 11 patients with recurrent hematomas, seven (63.6%) were ≥60 years old and four (36.4%) were <60 years old (P > 0.05).
Outcome
Historically, it has been known that older age, bilateral CSDH, low admission GCS score and recurrent hematomas are related to worse outcomes and increased mortality in patients undergoing surgical treatment for CSDH. Antunes et al. reported on a series of a 100 patients >75 years of age with a mortality rate of 15.6%.[4] This percentage is much higher than the mortality rates presented in other series, which did not address specifically the elderly population: 0.5%,[28] 1.5%,[27] 3.4%,[36] 4.4%[15] and 8%.[7] Yet, the author concluded that more than 60% of the patients were completely cured and that advanced age should not be considered a contraindication to surgery.[4] However, recurrence of CSDH was not associated with poor outcome and higher mortality rates in the publications of Ramachandran et al. and Gelabert-González et al.[26,14] Our study found that recurrence is associated with poor outcome at 6 months of follow-up (P = 0.0066).
Tsai et al. conducted a retrospective study comparing the outcome of bilateral and unilateral CSDH, and found that although the symptoms of increased intracranial pressure were more prominent in patients with bilateral hematomas, the postoperative outcome was similar to both groups.[31] In agreement with this article, our data did not show a statistically significant association between age or bilateral localization with outcome at 6 months.
Another significant factor determining outcome is the GCS score on admission.[2,3] Amirjamshidi et al. demonstrated in consecutive publications that lower admission GCS scores result in worse outcome, as this is measured by GOS, and higher mortality.[2,3] These results are in line with our data, suggesting that patients with an admission GCS score ≥9 present good outcome (P = 0.0033).
Limitations of the study
This study has some limitations. The major limitation is its retrospective nature. Yet, the authors’ standardized surgical approach and the consistent outcome evaluation at 6 months as a routine practice greatly facilitated the objective collection of data. Stratification by age in only two groups due to the limited number of patients could also raise some “oversimplification” issues. Of note, due to technical issues, the laboratory coagulations tests could not be retrieved. Thus, the role of abnormal coagulation status on final outcome could not be assessed. This series did not also analyze the relation between history of seizures or diabetes mellitus, width and architecture of the hematoma and postoperative drainage volume with recurrence. The authors have initiated a prospective study to face these issues, validate further and extend the findings of the current retrospective analysis.
CONCLUSION
Our data suggest that an admission GCS score ≥9 is associated with good outcome in patients who undergo surgical treatment for CSDH at 6 months postoperatively. On the contrary, recurrence is associated with poor outcome at the same period. Interestingly, our findings support the thesis that age is not associated with outcome in patients with CSDH. Thus, elderly patients could actually benefit from a surgical treatment, and they should be provided with that option. Finally, the presence of bilateral CSDH seems to have no relation with outcome or recurrence in these patients. This study could serve as an introduction to a more complex, multivariate debate specifically focused on the Brazilian population.
The authors report no conflict of interest related to this article.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/150/104744
Contributor Information
Danilo Otávio de Araújo Silva, Email: daniloncr@gmail.com.
Georgios K. Matis, Email: gkmatis@yahoo.gr.
Leonardo Ferraz Costa, Email: leosport2000@yahoo.com.br.
Matheus Augusto Pinto Kitamura, Email: matheus_kitamura@hotmail.com.
Eduardo Vieira de Carvalho Junior, Email: evcj2005@gmail.com.
Monalisa de Moura Silva, Email: monalisa@gmail.com.
Breno José A. P. Barbosa, Email: brenojb@gmail.com.
Carlos Umberto Pereira, Email: umberto@infonet.com.br.
Joacil Carlos da Silva, Email: jneurosurgery@gmail.com.
Theodossios A. Birbilis, Email: mpirmpil@otenet.gr.
Hildo Rocha Cirne de Azevedo Filho, Email: azevedoh@uol.com.br.
REFERENCES
- 1.Abouzari M, Armin R, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61:794–7. doi: 10.1227/01.NEU.0000298908.94129.67. [DOI] [PubMed] [Google Scholar]
- 2.Amirjamshidi A, Abouzari M, Eftekhar B, Rashidi A, Rezaii J, Esfandiari K, et al. Outcomes and recurrence rates in chronic subdural hematoma. Br J Neurosurg. 2007;21:272–5. doi: 10.1080/02688690701272232. [DOI] [PubMed] [Google Scholar]
- 3.Amirjamshidi A, Abouzari M, Rashidi A. Glasgow Coma Scale on admission is correlated with postoperative Glasgow Outcome Scale in chronic subdural hematoma. J Clin Neurosci. 2007;14:1240–1. doi: 10.1016/j.jocn.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Antunes DG, Alliez RJ, Eva L, Reynier Y, Alliez B. Analysis of the surgical treatment of chronic subdural hematoma in 100 elderly patients. Arq Bras Neurocir. 2006;25:156–60. [Google Scholar]
- 5.Arbit E, Patterson RH, Jr, Fraser RA. An implantable subdural drain for treatment of chronic subdural hematoma. Surg Neurol. 1980;15:175–7. doi: 10.1016/0090-3019(81)90133-6. [DOI] [PubMed] [Google Scholar]
- 6.Arseni C, Stanciu M. Particular clinical aspects of chronic subdural hematoma in adults. Eur Neurol. 1969;2:109–22. doi: 10.1159/000113778. [DOI] [PubMed] [Google Scholar]
- 7.Camel M, Robert L, Grubb JR. Treatment of chronic subdural hematoma by twist-drill craniostomy with continuous catheter drainage. J Neurosurg. 1986;65:183–7. doi: 10.3171/jns.1986.65.2.0183. [DOI] [PubMed] [Google Scholar]
- 8.Echlin FA, Sordillo SV, Garvey TQ., Jr Acute, subacute and chronic subdural hematoma. J Am Med Assoc. 1956;161:1345–50. doi: 10.1001/jama.1956.02970140001001. [DOI] [PubMed] [Google Scholar]
- 9.El-Kadi H, Miele VJ, Kaufman HH. Prognosis of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:553–67. [PubMed] [Google Scholar]
- 10.Ernestus RI, Beldzinski P, Lanfermann H, Norfrid K. Chronic subdural hematoma: Surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48:220–5. doi: 10.1016/s0090-3019(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 11.Fogelholm R, Heiskanen O, Waltimo O. Chronic subdural hematoma in adults.Influence of patient's age on symptoms, signs and thickness of hematoma. J Neurosurg. 1975;42:43–6. doi: 10.3171/jns.1975.42.1.0043. [DOI] [PubMed] [Google Scholar]
- 12.Fogelholm R, Waltimo O. Epidemiology of chronic subdural hematoma. Acta Neurochir (Wien) 1975;32:247–50. doi: 10.1007/BF01405457. [DOI] [PubMed] [Google Scholar]
- 13.Gastone P, Fabrizia C, Homere M, Francesco C, Alberto A, Nicola D. Chronic subdural hematoma: Results of a homogeneous series of 159 patients operated on by residents. Neurol India. 2004;52:475–7. [PubMed] [Google Scholar]
- 14.Gelabert-González M, Iglesias-Pais M, Garcia-Allut A, Martínez-Rumbo R. Chronic subdural hematoma: Surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107:223–9. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: The role of craniotomy reevaluated. Neurosurgery. 1993;31:67–72. doi: 10.1227/00006123-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Javadi A, Amirjamshidi A, Aran S, Hosseini SH. A randomized controlled trial comparing the outcome of burr-hole irrigation with and without drainage in the treatment of chronic subdural hematoma: A preliminary report. World Neurosurg. 2011;75:731–6. doi: 10.1016/j.wneu.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 17.Kotwica Z, Brezesinski J. Chronic subdural hematoma treated by burr holes and closed system drainage: Personal experience in 131 patients. Br J Neurosurg. 1991;5:461–5. doi: 10.3109/02688699108998474. [DOI] [PubMed] [Google Scholar]
- 18.Kwon TH, Park YK, Lim DJ, Cho TH, Chung YG, Chung HS, et al. Chronic subdural hematoma: Evaluation of the clinical significance of post-operative drainage volume. J Neurosurg. 2000;93:796–9. doi: 10.3171/jns.2000.93.5.0796. [DOI] [PubMed] [Google Scholar]
- 19.Markwalder TM, Seiler RW. Chronic subdural hematomas: To drain or not to drain? Neurosurgery. 1985;16:185–8. doi: 10.1227/00006123-198502000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Akagi K, Abekura M, Ryujin H, Ohkawa M, Iwasa N, et al. Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage. Neurol Res. 1999;21:277–80. doi: 10.1080/01616412.1999.11740931. [DOI] [PubMed] [Google Scholar]
- 21.Mckissock W, Richardson A, Bloom WH. Subdural hematoma.A review of 389 cases. Lancet. 1960;1:1365–9. [Google Scholar]
- 22.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001;41:371–81. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 23.Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and post-operative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791–5. doi: 10.3171/jns.2000.93.5.0791. [DOI] [PubMed] [Google Scholar]
- 24.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their post-operative recurrence. J Neurosurg. 2001;95:256–62. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 25.Probst C. Peritoneal drainage of chronic subdural hematomas in older patients. J Neurosurg. 1988;68:908–11. doi: 10.3171/jns.1988.68.6.0908. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran R, Hegde T. Chronic subdural hematomas: Causes of morbidity and mortality. Surg Neurol. 2007;67:367–72. doi: 10.1016/j.surneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Robinson RG. Chronic subdural hematoma: Surgical management in 133 patients. J Neurosurg. 1984;61:263–8. doi: 10.3171/jns.1984.61.2.0263. [DOI] [PubMed] [Google Scholar]
- 28.Sambasivan M. An overview of chronic subdural hematoma: Experience with 2300 cases. Surg Neurol. 1997;47:423–7. doi: 10.1016/s0090-3019(97)00188-2. [DOI] [PubMed] [Google Scholar]
- 29.Tabaddor K, Shulman K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed system drainage. J Neurosurg. 1997;46:220–6. doi: 10.3171/jns.1977.46.2.0220. [DOI] [PubMed] [Google Scholar]
- 30.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematomas: A review of 343 consecutive surgical cases. Neurosurgery. 2008;63:1125–9. doi: 10.1227/01.NEU.0000335782.60059.17. [DOI] [PubMed] [Google Scholar]
- 31.Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF. A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: Precipitating factors and postoperative outcomes. J Trauma. 2010;68:571–5. doi: 10.1097/TA.0b013e3181a5f31c. [DOI] [PubMed] [Google Scholar]
- 32.Wakai S, Hashimoto K, Watanabe N, Inoh S, Ochial C, Nagal M. Efficacy of closed system drainage in treating chronic subdural hematoma: A prospective comparative study. Neurosurgery. 1990;26:771–3. doi: 10.1097/00006123-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Wetch DA. A brief history of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:395–8. [PubMed] [Google Scholar]
- 34.Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: Results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98:1217–21. doi: 10.3171/jns.2003.98.6.1217. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda LC, Morita EM, Nishimori YF, Yasuda MA, Alves HA. Chronic subdural hematoma: Study of 161 patients and the relationship with coagulation abnormalities. Arq Neuropsiquiatr. 2003;61:1011–4. doi: 10.1590/s0004-282x2003000600023. [DOI] [PubMed] [Google Scholar]
- 36.Zumofen D, Regli L, Levivier M, Krayenbühl N. Chronic subdural hematomas treated by burr hole trepanation and a subperiostal drainage system. Neurosurgery. 2009;64:1116–22. doi: 10.1227/01.NEU.0000345633.45961.BB. [DOI] [PubMed] [Google Scholar]


