Figure 1.
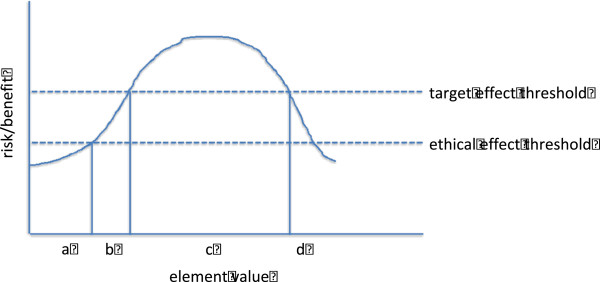
How early trials proceed with respect to a single dimension. Investigtors do not yet know the value for a given dimension, such as dose. (A) The investigators begin the study at a value that exceeds an ethical threshold (that is, value for which there is warranted belief that knowledge gain will redeem burdens and risks of drug administration). (B) Investigators escalate dimension values and cross a target effect threshold (which could be a pharmacokinetic variable, or a biological response of some sort). They have now defined the lower edges of dimension values. (C) They continue escalating and eventually re-cross a target effect threshold (D). When there are solid grounds for knowing they have crossed this target effect threshold, but well before re-crossing the ethical threshold, they discontinue escalation. The burdens or risks in (D) are considerably higher than elsewhere in the study, but enable warranted belief about the upper boundaries of therapeutic dimension values.
