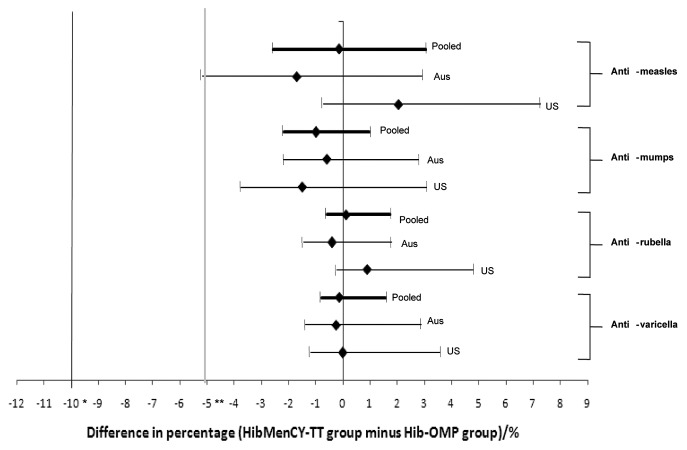
Figure 1. Difference between the HibMenCY-TT and Hib-OMP groups in percentage of subjects who seroconverted for measles, mumps, and varicella and with a seroresponse to rubella 42 d post-fourth dose of HibMenCY-TT/Hib-OMP in the Australian study (Aus), US immunogenicity cohort (US), and pooled analysis (Pooled) (ATP cohorts for immunogenicity; subjects who were seronegative at baseline). Limits represent 95% confidence intervals. Footnote: Anti-measles seroconversion: post-vaccination antibody concentration ≥ 150 mIU/mL in initially seronegative subjects (< 150 mIU/mL); anti-mumps seroconversion: post-vaccination antibody titer ≥ 28 ED50 in initially seronegative subjects (< 28 ED50); anti-rubella seroresponse: post-vaccination antibody concentration ≥ 10 IU/mL in initially seronegative subjects (< 4 IU/mL); anti-varicella seroconversion: post-vaccination antibody titer ≥ 1:5 in initially seronegative subjects (< 1:5); * limit of non-inferiority varicella; ** limit of non-inferiority measles, mumps, and rubella.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.