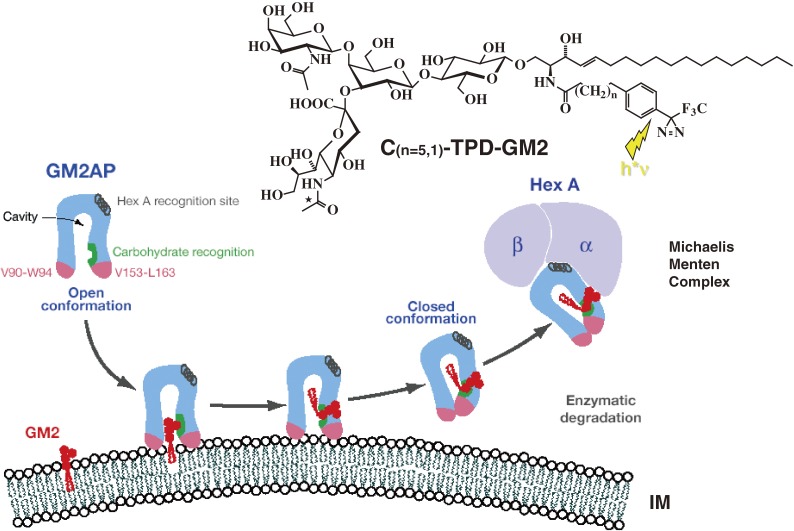
Figure 3.
Mechanism of GM2AP-Liftase. Model for the interaction of GM2-activator protein with luminal lysosomal membranes in the degradation of ganglioside GM2 (modified after ref. 36). GM2AP interacts with the membrane by dipping the two exposed hydrophobic loops, V90-W94 and V153-L163, into the apolar part of the membrane. Ganglioside GM2 is recognized by specific sites at the rim of the cavity. In the open protein conformation, the large hydrophobic area reaching from the apolar phase of the membrane to the activator’s cavity lowers the energy barrier for lipids leaving the membrane in an upward direction. After the ceramide tail has moved inside the activator’s cavity, the inward pointing orientation for the hydrophobic loop V153-L163 is favoured and the conformation changes to the closed form.254) This folding in of the hydrophobic loop leaves a more polar patch close to the membrane. The activator is then anchored only by the loop V90-W94. It may now rotate slightly upwards to expose all polar patches more fully to the solvent, and it may also leave the membrane and interact with the degrading enzyme. The photoaffinity label analogues C(n=5,1)-TPD-GM2 were photoincorporated specifically into the region V153-L163.