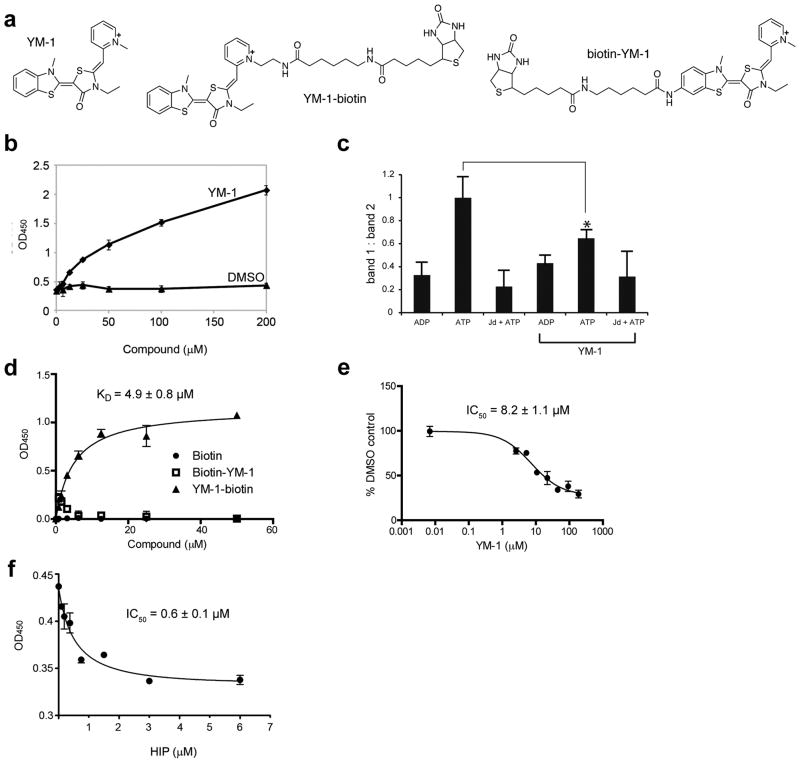
Figure 2. YM-1 increases Hsp70 binding to a denatured substrate and competes with Hip for binding.
(a) Chemical structures of YM-1 and the two biotinylated probes, 2 and 3. (b) YM-1 promotes binding of Hsp70 to denatured luciferase. Binding of purified Hsp70 to denatured luciferase was measured using an HRP-coupled, anti-Hsp70 antibody. A solvent control is shown for comparison. Data are mean± SEM.(c) Partial trypsin proteolysis shows that YM-1 favors an ADP-like conformation. Full length human Hsp70 was treated with ADP, ATP or ATP and a J domain (Jd). While proteolysis of the ADP-treated sample produces a single characteristic band (band 2), addition of ATP yields an additional fragment (band 1). The ratio of bands 1 and 2 are quantified and the error of duplicate experiments is shown. *P<0.005.(d) Binding of the biotinylated probes to Hsp70 confirms that the pyridine ring of YM-1 is solvent exposed. Experiments were carried out in triplicate. Data are mean ± SEM. (e) Binding of 2 to Hsp70 is specific and can be competed with unlabelled YM-1. Experiments were carried out in triplicate. Data are mean ± SEM.(f) YM-1 and Hip bind competitively to Hsp70. Binding of 2 to immobilized Hsp70 is diminished by pre-incubation with increasing amounts of Hip. Experiments were carried out in triplicate. Data are mean ± SEM.