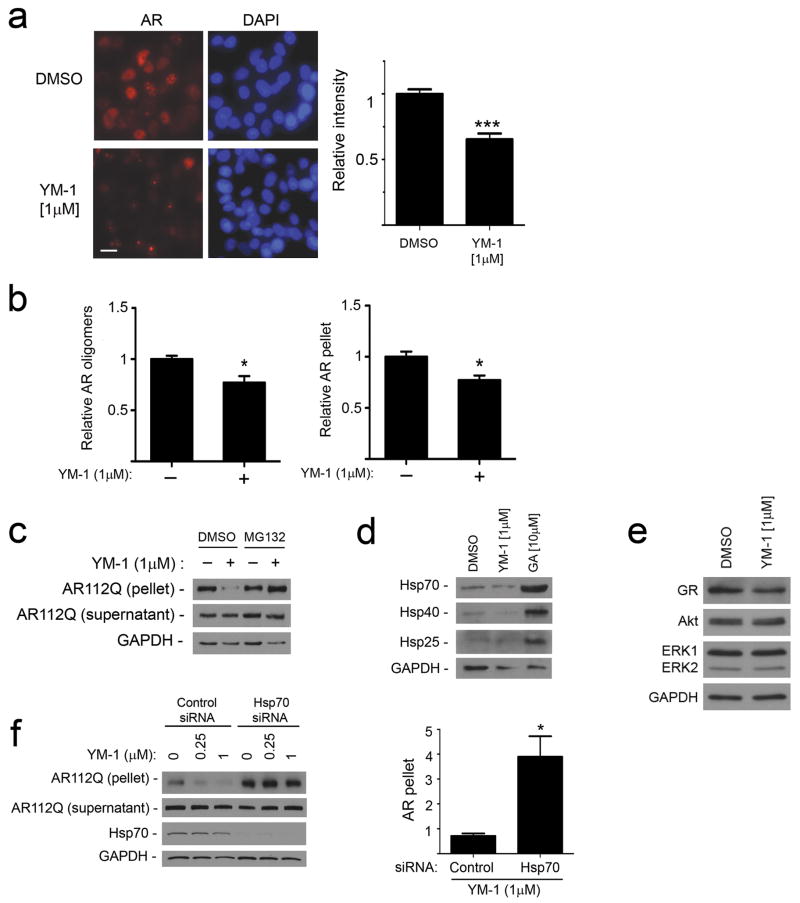
Figure 4. YM-1 increases Hsp70-dependent degradation of AR112Q.
(a) PC12 cells were induced to express AR112Q in the presence of R1881 (10 nM) for 48 hr, washed to remove doxycycline to turn off the transgene, and then incubated in the presence or absence of YM-1. Cells were stained for AR (left). Scale bar, 10 μm. Quantification of field signal intensity from three experiments (right). Data are mean ± SEM. ***P<0.001 (b) YM-1 promotes clearance of insoluble and oligomeric AR112Q. Signal intensity of AR112Q monomer in the 15,000 g pellet and high MW oligomers in the soluble fraction after ultracentrifugation was quantified from triplicate experiments (mean ± SEM). *P<0.05 (c) Immunoblot of AR112Q in supernatant and pellet shows that effects of YM-1 are blocked by 24 hr treatment with MG132 (10 μM). (For uncropped gel images, see Supplementary Figure 12.)(d) HeLa cells treated with vehicle, YM-1 or geldanamycin (GA) for 24 hr were probed for expression of inducible Hsp70, Hsp40 and Hsp25. (e) Levels of Akt and ERK1/2 are unchanged and glucocorticoid receptor (GR) mildly decreased by treatment of PC12 cells with YM-1, using the lysates probed in panel b.(f) YM-1 effects are dependent upon Hsp70. PC12 cells expressing tet-regulated AR112Q were transfected with siRNAs targeted at inducible Hsp70 or nontargeted control. After 24 hrs, cells were treated with R1881 (10 nM) plus YM-1 or vehicle. Effects of YM-1 on pelleted AR112Q are shown on left and quantified from triplicate experiments on right. Data are mean ± SEM. *P<0.05.