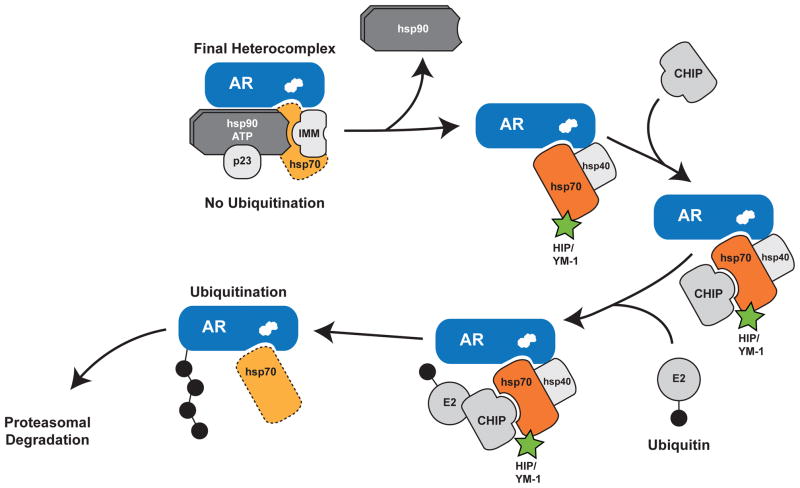
Figure 6. Model of the Hsp90/Hsp70-based chaperone machinery and regulation of polyQ AR degradation.
Hsp90 and Hsp70 form a heterocomplex to stabilize the polyQ AR, enable ligand binding (depicted as white steroid within AR) and guide intracellular localization (top left). Dissociation of Hsp90 following the addition of small-molecule inhibitors or ligand-dependent conformation change of the polyQ AR permits unfolding of the mutant protein. Substrate-bound Hsp70 then recruits chaperone dependent ubiquitin ligases such as CHIP to promote degradation through the proteasome. We note that CHIP and Hip both bind Hsp70 via tetratricopeptide repeat domains, although it is unknown whether this binding occurs simultaneously. Furthermore, we note that other chaperone dependent ubiquitin ligases may function redundantly with CHIP. We demonstrate here that allosteric activators of Hsp70, including Hip and YM-1 (in green), increase substrate binding affinity, facilitate client protein ubiquitination and promote polyQ AR clearance by the proteasome. This strategy alleviates polyglutamine toxicity by facilitating degradation of the mutant protein. The broken line for Hsp70 in the final heterocomplex indicates that it is present in substoichiometric levels with respect to the receptor. IMM, immunophilin.