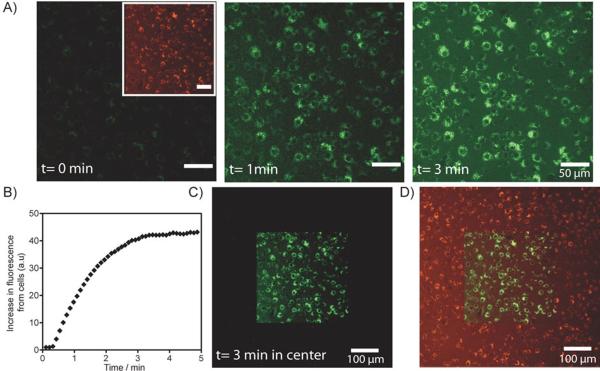
Figure 2.
Spatiotemporally controlled dual-color labeling of macrophage RAW cells. A) RAW cells were incubated with PANP and washed before imaging. Left inset, a CLSM image of RAW cells before photoactivation, where the entire population of PANP-labeled RAW cells are visible in the VT680 channel (pseudo-colored red). Left, prior to photoactivation (t=0 min), no fluorescence signal was detectable in the fluorescein channel. Center and right, fluorescein fluorescence of PANP was activated by a 405 nm laser light on the confocal microscope. Increasing light exposure lead to an increase in the fluorescein signal from cells (pseudo-colored green), detectable from the CLSM images acquired after 1 min and 3 min. Microscopic photoactivation was performed by sequential line scanning with the 405 nm diode laser, where exposure time per voxel (~1 μm3) was set at 10 μs per image. The total exposure time to 405 nm light of ~1 μm3 is ~100 μs (for image acquired at 1 min) and ~300 μs (for image acquired at 3 min). B) Increase in fluorescence in the fluorescein channel is plotted against image acquisition time. Continuous scanning of the cells with 405 nm light leads to an increase in fluorescein signal of the RAW cells over time till saturation is reached after about 3 min of image acquisition. C) Spatially restricted activation of PANP. Here, the activated region was imaged under lower magnification. The CLSM image shows cells with fluorescein fluorescence restricted to the photoactivated region of the imaging slide (central square). D) A merged CLSM image showing fluorescent signals from both the fluorescein and VT680 channels. The light exposed area (central square) contains dual-color labelled RAW cells.