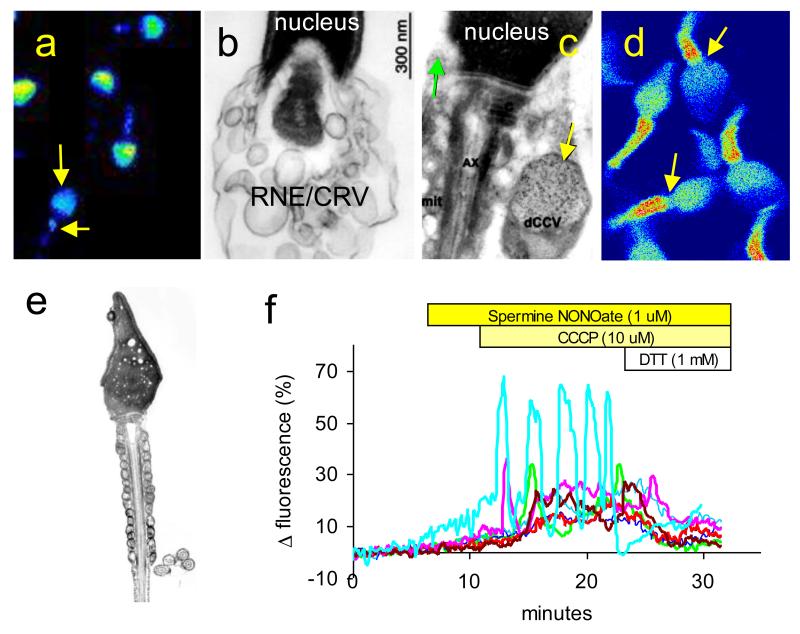
Figure 2.
Intracellular Ca2+ storage sites in mammalian sperm. a: Pseudocolour image of MagFluo-4 loaded sperm showing (warm colours show high [Ca2+]). This low-affinity Ca2+ indicator highlights the high concentrations of Ca2+ in the acrosomal store and at the sperm neck/midpiece (arrows). b: Electron micrograph showing posterior of the nucleus and the cytoplasmic droplet of a bovine sperm. Large numbers of membranous cisternae (parts of RNE and/or a discrete population of calreticulin containing vesicles – RNE/CRV) can be seen in this region. c: Electron micrograph showing posterior of the nucleus and the cytoplasmic droplet of a human sperm. Section has been labelled with gold-conjugated anti-calreticulin antibodies. Green arrow shows small calreticulin-containing vesicle in anterior of cytoplasmic droplet. Yellow arrow shows large calreticulin-containing vesicle in the cytoplasmic droplet adjoining the midpiece. d: Pseudocolour confocal image of Fluo-3 labelled human sperm (warm colours show high [Ca2+]). In cells from this donor there was a concentration of fluorescence in the mitochondrial midpiece, occurring as two ‘stripes’ of highly fluorescent points arranged exactly as the mitochondria appear in electron microscope sections (e). There is a clear gap (arrows) between the anterior end of the fluorescent ‘stripe’s and the sperm head, which is the position of the RNE and calreticulin-containing vesicles. f: Ca2+ oscillations at the sperm neck/midpiece do not require the mitochondrial membrane potential. Plots shows fluorescence from seven human sperm loaded with the Ca2+ indicator Oregon Green BAPTA 1. The NO• donor spermine NONOate causes protein S-nitrosylation, which sensitises the Ca2+ store in the neck/midpiece causing a slow elevation of [Ca2+]i (Machado-Oliveira et al, 2008). Upon application of the mitochondrial uncoupler CCCP, to collapse the mitochondrial inner membrane potential, there is a pronounced rise in [Ca2+]i (probably due to release of mitochondrial Ca2+) which is then followed in some cells by Ca2+ transients and oscillations (green, pink, brown and turquoise traces) in the sperm neck/midpiece. These transients and oscillations reflect cyclical release and re-uptake of Ca2+ stored at the sperm neck/midpiece by a mechanism that does not require an intact mitochondrial membrane potential. When dithiothreitol (DTT) is applied, reversing protein S-nitrosylation and removing the sensitising effect of NO on the Ca2+ store (Machado-Oliveira et al, 2008), the cells recover despite the continued presence of the mitochondrial uncoupler. Panel b from Ho and Suarez (2003) with permission, panel c from Naaby-Hanset et al (2001) with permission.