Figure 1.
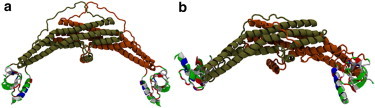
(a) A full endophilin protein was initially prepared by placing the N-terminal helix into the peptide-binding region of the SH3 domain with the hydrophobic residues of the helix pointed toward the groove to hide them from the solvent. (b) The protein was relaxed in solution for 101 ns and the H0/SH3 complex was found to be stable. The interaction between the helix and the SH3 domain was found to keep the SH3 domains at the distal end of the BAR domain and keep hydrophobic residues on both domains hidden from the solvent. The proteins are shown here in the New Cartoon representation. The BAR domain and linker are colored according to the protein chain, and the H0/SH3 complex is colored according to the residue type (hydrophobic residues are white, polar residues are green, acidic residues are red, and basic residues are blue).
