Figure 2.
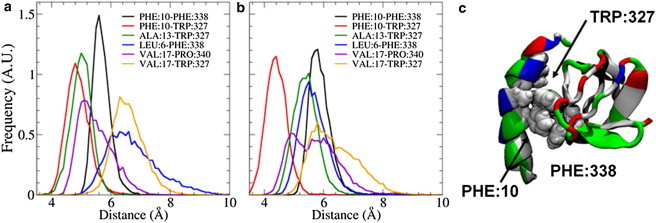
(a and b) The distributions of distances between hydrophobic residues on the helix and SH3 domain for the first (a) and second (b) endophilin monomer chains show that the strongest interactions exist between the Phe10 residue on the helix and residues Trp327 and Phe338 on the SH3 domain. (c) Inspection of the equilibrated H0/SH3 complex shows that the Trp327 and Phe338 residues form a pincer-like structure around the Phe10 residue, leading to a strong interaction. The H0/SH3 complex is shown using the New Cartoon and van der Waals representations and is colored according to residue type (hydrophobic residues are white, polar residues are green, acidic residues are red, and basic residues are blue).
