Figure 5.
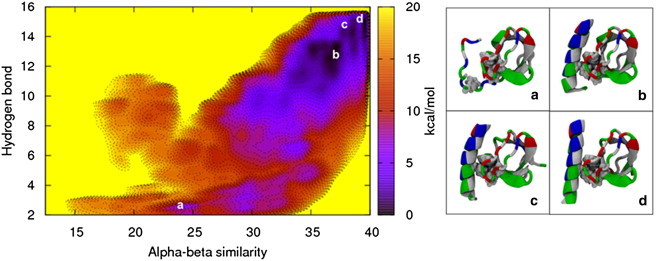
The folding free-energy landscape of the H0/SH3 complex in solution shows that the presence of the SH3 domain stabilizes the N-terminal helix. (a–d) The free-energy minimum (d), along with the other energetically stable folded states (b and c), keeps the Phe10 residue on the helix hidden from the solvent, whereas the unfolded state (a) exposes the residue to the solvent, resulting in a higher free-energy state. The H0/SH3 complex is shown using the New Cartoon and Van der Waals representations and is colored according to residue type (hydrophobic residues are white, polar residues are green, acidic residues are red, and basic residues are blue).
