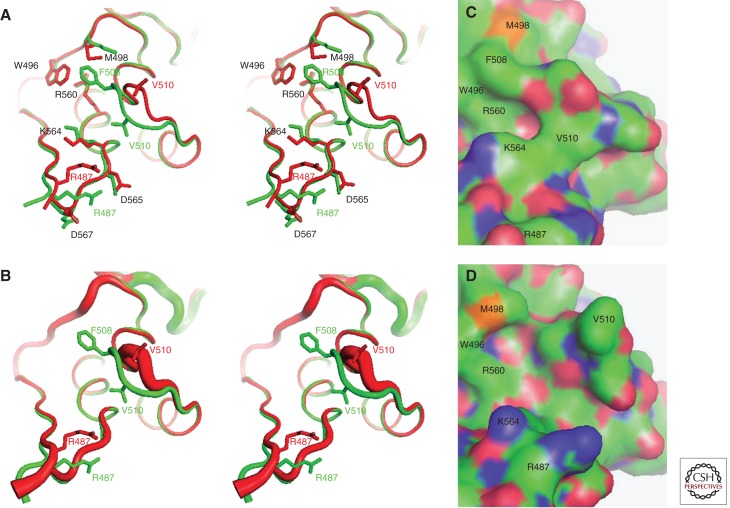
Figure 4.
Structural consequences of the F508del mutation in hNBD1. Structures of hNBD1 with and without the F508del mutation were aligned based on least-squares superposition of the three α-helices comprising the core of the ABCα subdomain. (A) Stereopair showing the interface between the F1-like core subdomain and the ABCα subdomain in F508 (green, PDB ID 2BBO) and F508del (red, PDB ID 1XMJ) hNBD1 structures. Selected sidechains are shown in ball-and-stick representation. The site of attachment of hNBD1 to the transmembrane domains of CFTR is proximal to the viewer in this orientation, and the γ-PS is at the upper left (i.e., the backbone segment including residues W496 and M498). (B) Stereopair showing the same view of the same hNBD1 structures using the same color scheme, but with the thickness of the backbone traces encoding their mean backbone B-factors (using the default scheme in PyMOL, with 0.6–4.0 Å radius representing 33–77 Å2 and 12–69 Å2 for the F508 and F508del structures, respectively). (C,D) Surface representations of the same structures showing the alterations in local surface topography caused by the F508del mutation. These two panels show the same view of the molecular surfaces of F508 (panel C) and F508del (panel D) hNBD1, with carbon atoms colored green, oxygen atoms colored red, nitrogen atoms colored blue, and sulfur atoms colored orange. Note that the structures shown here contain seven point mutations included in hNBD1 constructs because of their beneficial influence on yield during purification—F409L, F429S, F433L, G550E, R553Q, R555K, and H667R. Extensive analyses led to the conclusion that these mutations do not significantly perturb the native ground-state conformation of hNBD1 (Lewis et al. 2010) but that they do stabilize it thermodynamically compared to an aggregation-prone molten globule intermediate that accounts for both the instability of F508del-CFTR in vivo and the progressive loss of hNBD1 constructs in vitro during purification (Protasevich et al. 2010; Wang et al. 2010). (The images shown here are from Lewis et al. 2010; adapted, with permission, from the authors.)