Fig. 4.
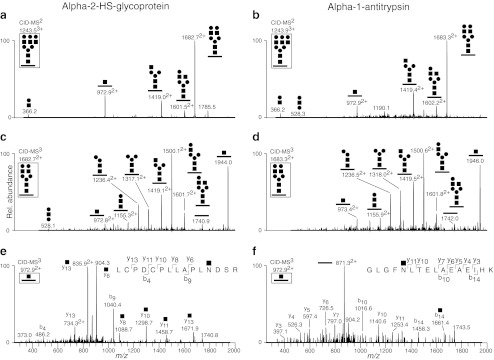
FTICR-MS/CID-MSn analysis of N-glycopeptides enriched from bovine serum. a CID-MS2 of the triantennary N-glycopeptide LCPDCPLLAPLNDSR of bovine alpha-2-HS-glycoprotein. b CID-MS2 of the triantennary N-glycopeptide GLGFNLTELAEAEIHK of bovine alpha-1-antitrypsin. c and d show CID-MS3 of the most abundant fragment ion in (a) and (b), respectively. e and f show CID-MS3 of the Y1-type (m/z 972.9) fragment in (a) and (b). The CID-MS3 spectra of the most abundant fragment ions at m/z 1682.7 (a) and m/z 1683.3 (b) were used to verify the N-glycan structure. The N-glycopeptide identities were verified only when the fragment ions at m/z 972.9 were analyzed by CID-MS3. The structure of precursor ions subjected to CID-MSn fragmentation are shown boxed in each panel
