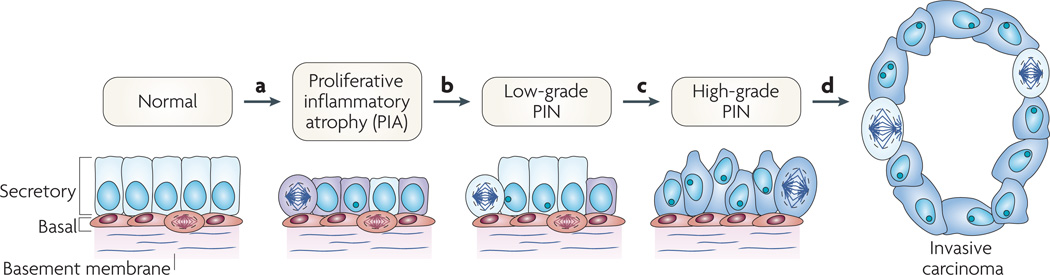
Figure 3. Cellular and molecular model of early prostate neoplasia progression.
a | This stage is characterized by the infiltration of lymphocytes, macrophages and neutrophils (caused either by repeated infections, dietary factors and/or by the onset of autoimmunity); phagocytes release reactive oxygen and nitrogen species causing DNA damage, cell injury and cell death, which trigger the onset of epithelial cell regeneration. The morphological manifestation of the cellular injury is focal prostate atrophy, which is proposed to signify the ‘field effect’ in the prostate. The downregulation of p27, NKX3.1 and phosphatase and tensin homologue (PTEN) proteins in luminal cells stimulates cell-cycle progression. Stress-response genes are induced (such as glutathione S-transferase P1 (GSTP1), GSTA1 and cyclooxygenase 2 (PTGS2)). b | The subsequent silencing of GSTP1 through promoter methylation in subsets of cells further facilitates oxidant-mediated telomere shortening. c | Cells carrying methylated GSTP1 alleles and short telomeres have dysfunctional telomeres and are more likely to bypass the senescence checkpoints. This favours the onset of genetic instability and the consequent accumulation of genetic changes (for example loss of heterozygosity on 8p21,6q or gain of function on 8q24,17q). d | The continued proliferation of genetically unstable luminal cells and the further accumulation of genomic changes, such as gene rearrangements leading to TMPRSS2–ETS family member gene fusions, lead to progression towards invasive carcinomas. PIN, prostatic intraepithelial neoplasia.