Abstract
The arms and legs of man are evolutionarily derived from the paired fins of primitive jawed fish. Few evolutionary changes have attracted as much attention as the origin of tetrapod limbs from the paired fins of ancestral fish. The hindlimbs of tetrapods are derived from the pelvic fins of ancestral fish. These evolutionary origins can be seen in the examination of shared gene and protein expression patterns during the development of pelvic fins and tetrapod hindlimbs. The pelvic fins of fish express key limb positioning, limb bud induction and limb outgrowth genes in a similar manner to that seen in hindlimb development of higher vertebrates. We are now at a point where many of the key players in the development of pelvic fins and vertebrate hindlimbs have been identified and we can now readily examine and compare mechanisms between species. This is yielding fascinating insights into how the developmental programme has altered during evolution and how that relates to anatomical change. The role of pelvic fins has also drastically changed over evolutionary history, from playing a minor role during swimming to developing into robust weight-bearing limbs. In addition, the pelvic fins/hindlimbs have been lost repeatedly in diverse species over evolutionary time. Here we review the evolution of pelvic fins and hindlimbs within the context of the changes in anatomical structure and the molecular mechanisms involved.
Keywords: development, evolution, hindlimb, pelvic fin
Introduction to pelvic fins and hindlimbs
The transition of vertebrates from water to land was one of the greatest steps in evolutionary history. This required the development of paired pelvic fins and their muscles to eventually form weight-bearing hindlimbs (Goodrich, 1930). A number of fossil forms have shed light on the evolution of pelvic fins and hindlimbs within ancestral fish and early tetrapods (Andrews & Westoll, 1970; Coates, 1996; Jarvik, 1996; Clement et al. 2004; Ahlberg et al. 2005, 2008; Boisvert, 2005; Callier et al. 2009; Niedzwiedzki et al. 2010; Zhu et al. 2012). Studies of these fossils have shown that the transition from paired pelvic fins to tetrapod hindlimbs is characterised by a gradual progression from posterior and ventrally placed slender pelvic fins articulated to a pelvic girdle to a dorsally located robust pelvis and hindlimb. Recent genetic approaches have led us to a point where the key developmental mechanisms have been identified in pelvic fins and hindlimbs and we are now able readily to compare between species. We will be presenting evidence for the evolution of pelvic fins and hindlimbs organised by integration of anatomy and molecular mechanisms.
Pelvic fin and hindlimb morphology
During the evolutionary history of vertebrates, paired pelvic appendages have undergone many changes since their first appearance. In fish, pelvic fins vary greatly in morphology, function and position, whereas in tetrapods they have evolved into robust weight-bearing hindlimbs necessary for terrestrial locomotion.
Although the morphology of the fish fin differs greatly from that of the tetrapod limb, a clear connection in the evolution of limbs from fins can be seen in the anatomy of the paired fins of chondrichthyans, actinopterygians, sarcopterygians and the limbs of tetrapods (Janvier, 1996). The pelvic fin of chondrichthyans (sharks and rays) is composed of a propterygium, a mesopterygium and a metapterygium. Whereas basal actinopterygians (e.g. paddlefish) have maintained all three elements of the pelvic fin, teleosts (e.g. zebrafish) have maintained the propterygium and mesopterygium, and have lost the metapterygium (Coates, 1994; Coates & Cohn, 1998) and sarcopterygians (lobe-finned fish) have maintained the metapterygium. The tetrapod limb is thought to originate from the metapterygium of sarcopterygians (reviewed in Wagner & Chiu, 2001) (Fig. 1).
Fig. 1.
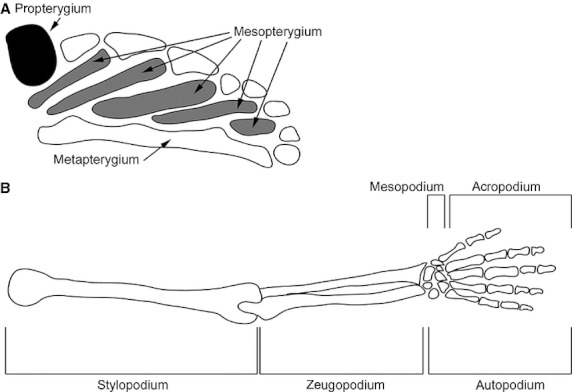
Schematic representation the appendicular endochondral skeleton of a basal fish and a tetrapod. (A) A Polyodon (paddlefish) pectoral fin (adapted from Grande & Bemis 1991). (B) A human arm. Each appendage is orientated with anterior (preaxial) upward and distal to the right. Figures are not to scale. Reproduced with permission from Wagner & Larsson (2007) and Grande & Bemis (1991).
The pelvic fins of chondrichthyans and basal ray-finned fish are usually located at an approximately mid-body position which is posterior to the centre of mass. The pelvic girdle of sharks is embedded in the body wall and is articulated to a number of elongated cartilaginous elements, which support the pelvic fin rays (Liem & Summers, 1999).
The distinctive pelvic fins of teleosts consist of three main skeletal elements; the basipterygium (pelvic girdle), a reduced number of radials (fin base) and the lepidotrichs (slender bony fin rays) (Stiassny & Moore, 1992; Cubbage & Mabee, 1996; Coates & Cohn, 1998). The pelvic fins are supported by a cartilaginous endoskeleton which ossifies during development. The endoskeleton consists of a pelvic girdle which articulates with the endoskeleton of the pelvic fin, the pattern of which differs greatly between species (Goodrich, 1930; Zangerl, 1981; Shubin, 1995). The pelvic fins of fish consist of a proximal muscular component and a distal non-muscularised dermal fin fold. The fin folds are supported by long fin rays which are ossified (Grandel & Schulte-Merker, 1998). Teleosts usually have six pelvic fin muscles, three pairs lying on each side of the pelvis (Winterbottom, 1974). The arrector ventralis pelvicus, the abductor superficialis pelvicus and the abductor profundus pelvicus are found on the ventral side of the pelvic girdle. The arrector dorsalis pelvicus, adductor superficialis pelvicus and the adductor profundus pelvicus are located on the medial side of the pelvic girdle. Infracarinalis anterior is connected to the basipterygium and the cleithrum, while the infracarinalis medius is connected to the basipterygium and the first anal-fin pterygiophores. The extensor proprius is not always present but can be found on the dorsal side of the girdle (Winterbottom, 1974; Stiassny & Moore, 1992) (Fig. 2). The positioning of the adult pelvic fin has shifted during teleost fish evolution, ranging from an abdominal position in the ventral body wall near the cloacae to an anterior position at either a thoracic or a jugular level in more derived teleost groups, although there are many exceptions to this condition (Greenwood et al. 1966; Rosen, 1982; Nelson, 1994).
Fig. 2.
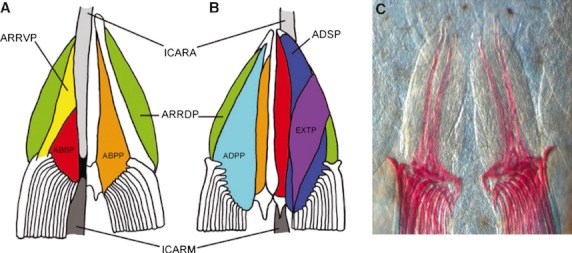
Schematic diagram and bone and cartilage staining of the musculature of the pelvic girdle of teleost. Ventral (A, C) and dorsal (B) views of the pelvic fin muscles (anterior to the top). ABSP, abductor superficialis pelvicus (red); ABPP, abductor dorsalis pelvicus (orange); ADPP, adductor profundus pelvicus (light blue); ADSP, adductor superficialis pelvicus (blue); ARRDP, arrector dorsalis pelvicus (green); ARRVP, arrector ventralis pelvicus (yellow); EXTP, extensor proprius (purple); ICARA, infracarinalis anterior (light grey); ICARM, infracarinalis medius (dark grey). A tendon is shown in black. Reproduced with permission from Winterbottom (1974); and Yamanoue et al. (2010); and modified with permissions from Don et al. (2011).
Amongst teleosts, there are many exceptional pelvic fin structures such as dorsally placed pelvic fins (Bathophilus, Fink, 1985), unpaired structures (such as the fused pelvic spine of triggerfish and the dewlap of filefishes; Matsuura, 1979; Tyler, 1980) and even the complete loss of pelvic fins (some sticklebacks and some zebrafish, Cole et al. 2003; Shapiro et al. 2004; Chan et al. 2010; Don et al. 2011). It is possible that as the pelvic fins are not absolutely necessary for survival, this diverse range of morphologies is possible.
The pelvic fins of lobe-finned fish are the evolutionary forerunners of the hindlimbs of tetrapods. The pelvic fins of lobe-finned fish are derived from the metapterygium of chondrichthyans and basal actinopterygian fish (Mabee, 2000; Raff, 2007). The pelvic girdle of lobe-finned fish articulates with the femur; at its distal tip, the femur articulates with the tibia and fibula, which may be fused in basal sarcopterygians (Vorobyeva & Hinchliffe, 1995). The mesopodium of lobe-finned fish is composed of an incomplete and variable arrangement of bones, which makes up the ankle in tetrapods (Wagner & Chiu, 2001). The more distal acropodium is absent in the pectoral and pelvic fins of living and extinct sarcopterygian fish (Johanson et al. 2007). The muscles of the pelvic fin of an extant sarcopterygian fish, the Australian lungfish (Neoceratodus forsteri) have been described previously (Young et al. 1989 and Boisvert et al. 2009). The adductor muscles of the Australian lungfish pelvic fin are the superficial ventromesial adductor, the superficial ventrolateral adductor, the deep ventral adductor depressor, the dorsomesial adductor levator and the mesial adductor (Young et al. 1989; Boisvert et al. 2009). The pelvic fin abductor muscles of the Australian lungfish are the superficial ventromesial abductor, the superficial ventrolateral abductor, the deep ventral abductor depressor and the dorsolateral abductor levator (Young et al. 1989; Boisvert et al. 2009). The radial flexors of the lungfish pelvic fin functions as both an adductor and abductor (Young et al. 1989; Boisvert et al. 2009). The supinator and pronator pelvic fin muscles of the Australian lungfish are possibly the precursors to tetrapod digit muscles as the radial–axial muscles of the Australian lungfish attach to the distal radials and it is thought that the distal radials of ancient fish were the precursors of digits (Young et al. 1989; Johanson et al. 2007; Boisvert et al. 2008, 2009). The hypaxial muscles of the Australian lungfish do not attach to the pelvis (Young et al. 1989; Boisvert et al. 2009).
Fossil evidence suggests that tetrapod hindlimb originated in the Devonian 370 million years ago and that it has its origins in the metapterygium of sarcopterygians (Vorobyeva, 1991; Ahlberg & Milner, 1994; Wagner & Chiu, 2001). Most tetrapod hindlimbs follow a general plan of a proximal stylopodium, a zeugopodium and a distal autopodium. The stylopodium of modern tetrapods consists of a single elongated skeletal element called the femur. The modern tetrapod zeugopodium consists of two parallel skeletal elements, the fibula and the tibia. The autopodium of modern tetrapods consists of the more proximal mesopodium and the distal acropodium. A full complement of tarsal bones makes up the mesopodium or the ankle, whereas the more distal acropodium usually consists of five radiating digits (Fig. 1B). The pelvis of modern tetrapods is made up of three elements, the ilium, ischium and pubis, and is fused to the vertebral column though a sacral rib (Clack, 2000). The muscles of the salamander (Necturus maculosus) hindlimb have been previously described and compared with the pelvic fin muscles of the Australian lungfish (Young et al. 1989; Walker & Homberger, 1992; Boisvert et al. 2009). Most of the muscles of the salamander hindlimb and pelvic girdle can be matched in both insertion points and function to their equivalents in the Australia lungfish. The developmental mechanisms of how the pelvic fin muscles of ancient fish evolved to become more robust musculature of the tetrapod hindlimb will be discussed below.
Pelvic fin and hindlimb function
In addition to the varied morphology of pelvic fins and tetrapod hindlimbs, the paired pelvic appendages have a variety of functions. The slender pelvic fins of fish mostly play minor roles during swimming and manoeuvring, and the hindlimbs of tetrapods are usually employed as the major form of locomotion.
Dominate propulsion by the body and caudal fin during swimming is a common feature throughout the evolution of fish; however, the pelvic fins do play a role during steady swimming and manoeuvres, especially in teleosts (Gosline, 1980; Webb, 1982; Standen, 2008). Early work on the function of pelvic fins in dogfish concluded that the pelvic fins had a very limited and mostly passive, stabilising function during locomotion and were mainly concerned with the production of vertical forces (Harris, 1937, 1938). It was shown that, in sharks, the pelvic fins increase the static stability for pitching movements, but only to a small extent (Harris, 1938).
In more derived ray-fin fishes, the pelvic fins have moved to beneath the centre of mass and have a greater degree of mobility when compared with the pelvic fins of sharks (Harris, 1938; Gosline, 1980; Rosen, 1982; Schrank et al. 1999). Early studies suggested that this adaptation leads to the pelvic fins of bony fish having little effect on body yaw when used simultaneously but being capable of inducing rolling movements (Harris, 1938; Gosline, 1980; Rosen, 1982; Schrank et al. 1999). More recent studies have suggested that the pelvic fins of teleost play an even greater role during swimming (Drucker & Lauder, 2003; Lauder & Drucker, 2004; Standen, 2008). It has been shown that teleosts actively use their pelvic fins as control surfaces during turning manoeuvres and in combination with other fins (anal and dorsal) compensate for pitching movements during breaking (Drucker & Lauder, 2003; Lauder & Drucker, 2004). A recent three-dimensional kinematics study of the rainbow trout (Oncorhynchus mykiss) has shown that during steady swimming, pelvic fins oscillate in regular contralateral cycles and during manoeuvres act as trimming foils. The cyclic oscillation, involving active and passive components, may function to dampen body oscillation and stabilise body position (Standen, 2008). When acting as trimming foils during manoeuvres, the pelvic fins move variably, which helps to stabilise and return the body to a steady swimming posture (Standen, 2008). Despite the new-found roles for the pelvic fins of teleosts during swimming and manoeuvring, the pelvic fins are still considered to be the least important fin for swimming because they have been lost frequently during evolution and their amputation does not greatly change body motion during swimming (Harris, 1938; Gosline, 1980; Standen, 2008).
There are also cases where the pelvic fins of teleosts are used for different kinds of locomotion. Some fish groups use their pelvic fins to move, as if walking over aquatic and terrestrial substrata (Peters, 1985; Webb, 1996), whereas others use hypertrophied pelvic fins, in combination with pectoral fins to fly (Davenport, 1992, 1994, 2003).
Although tetrapods display a large diversity of function in the hindlimb, most often, the robust weight-bearing hindlimbs of tetrapods are used for hindlimb-propelled locomotion. Most modern tetrapods predominantly employ hindlimb-powered symmetrical gaits such as lateral sequence walking where the hindlimb footfall is followed by the ipsilateral forelimb (Rewcastle, 1981). The hindlimb-propelled walk of most modern tetrapods is facilitated by several features unique to tetrapods, such as a weight-bearing pelvis that is attached to the vertebral column, a laterally orientated leg, an elongated femur for longer stride length and a flexible ankle. It is important to note that not all tetrapods use the hindlimb only for locomotion, examples of other functions of hindlimbs in tetrapods are the prehensile foot of monkeys and apes, and the loss of hindlimbs in cetaceans (whales and dolphins) for streamlined swimming (Fleagle, 1999; Bejder & Hall, 2002). It is possible that the variety of functions and morphologies of the tetrapod hindlimb is more constricted than in the pelvic fins of fish, as the robust weight-bearing hindlimb of most tetrapod provides locomotion which is necessary for survival. However, the vast array of various morphologies and functions reflect the evolutionary changes that have occurred in pelvic fins and hindlimbs since their first appearance.
Evolution of pelvic fins and hindlimbs
Origin of paired limbs
For over a century the origin of vertebrate paired fins and limbs has been fiercely debated. One of the first theories put forward proposed that fins evolved from the gill arches of the early limbless vertebrates (Gegenbaur, 1878). Recent theory proposes that the fins of vertebrates evolved from continuous stripes of competency for appendage formation located ventrally and laterally along the embryonic flank (Yonei-Tamura et al. 2008) (Fig. 3A). A continuation of this theory proposed that the paired appendages of jawed vertebrates evolved with a shift in the zone of competency to the lateral plate mesoderm (LPM) in conjunction with the establishment of the lateral somitic frontier, which allowed for the formation of limb/fin buds with internal supporting skeletons (Freitas et al. 2006; Durland et al. 2008; reviewed Johanson, 2010) (Fig. 3A). The conservation of genetic mechanisms (Hox and Tbx expression patterns) between median fins and paired fins of shark and lamprey embryos supports this theory (Freitas et al. 2006).
Fig. 3.
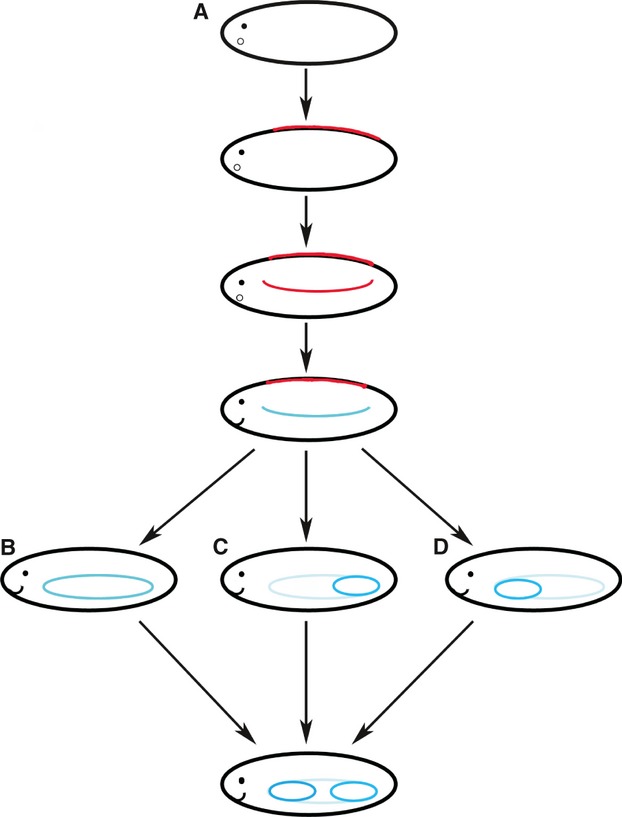
Diagram of the evolution of paired fins. (A) The evolution of paired fins from the ‘zone of competence’ (Freitas et al. 2006; Yonei-Tamura et al. 2008; Johanson, 2010). A dorsal zone of competence for unpaired fins is the first to arise in jawless vertebrates (Johanson, 2010). The dorsal zone of competence was then duplicated and co-opted to a ventrolateral position along the flank (Freitas et al. 2006). The ventrolateral zone of competence was then shifted to the LPM, which coincided with the evolution of the abaxial region and the lateral somitic frontier (Johanson, 2010). (B) The ‘lateral fin fold’ theory suggests that two paired fins evolved from a single continuous lateral fin (Thacher, 1877; Mivart, 1879; Balfour, 1881). (C) Based on the collinear expression pattern of Hox gene expression, the ‘pelvic before pectoral’ theory suggests that the pelvic fins evolved before the pectoral fins (Tabin & Laufer, 1993). (D) Based on fossil evidence and the anterior–posterior pattern of development, the ‘pectoral before pelvic’ theory suggests that pectoral fins evolved first and were then duplicated to form pelvic fins (Coates, 1993; Thorogood & Ferretti, 1993).
To explain the emergence of ‘two sets’ of paired fins, several theories have been put forward. The Thacher–Mivart–Balfour fin fold theory of the origin of the paired fins suggests that tetrapod forelimbs and hindlimbs evolved by the splitting of a single lateral fin (Thacher, 1877; Mivart, 1879; Balfour, 1881) (Fig. 3B). This theory has been contested due to the inconsistency with the fossil record and a lack of embryonic evidence. In contrast, Tabin & Laufer (1993) suggested that pelvic fins were acquired before pectoral fins in the ‘pelvic before pectoral’ fin model due to the collinear expression pattern of the Hox genes along the embryonic flank and in developing limb buds (Fig. 3C). It was thought that the pattern of Hox gene expression along the flank of the embryo was co-opted into the pelvic fins and then passed to the pectoral fins, which is why only the posterior Hox genes are expressed during limb/fin development (Tabin & Laufer, 1993). However, to date, no fossils have been described which possess only pelvic fins (Coates, 1993; Thorogood & Ferretti, 1993) and these authors suggested that based on fossil and developmental evidence that pectoral fins were acquired before pelvic fins (Fig. 3D). Ruvinsky & Gibson-Brown (2000) proposed that an ancestral Tbx4/5 cluster was initially co-expressed in the first pair of fins to evolve. In modern jawed vertebrates, Tbx4 is expressed in the pelvic appendages and Tbx5 is expressed in the pectoral appendages (Gibson-Brown et al. 1996; Tamura et al. 1999; Ruvinsky et al. 2000). To explain this expression pattern, it was suggested that the ancestral Tbx4/5 cluster underwent a duplication event, either before or Tbx4 became localised at the pelvic level or after the cluster became localised at the pelvic level (Ruvinsky & Gibson-Brown, 2000). In this model, Tbx4 acted in conjunction with Pitx1 to modify the morphology of the developing limb to a pelvic fin/hindlimb identity (Ruvinsky & Gibson-Brown, 2000).
Origin of pelvic fins
Although the nature of the origin of paired fins remains controversial, it is now clear from the fossil record that pelvic fins arose within jawed fish (Fig. 4). Zhu et al. (2012) recently re-examined a fossil of a primitive antiarch, Parayunnanolepis xitunensis, from the early Devonian (approximately 430 Mya), which provided evidence for the presence of pelvic girdles in these phylogenetically basal placoderms (Zhu et al. 2012). Antiarch placoderms (extinct armoured jawed fishes) first appeared in the Silurian and as antiarchs are placed at the base of the gnathostome radiation, the far-reaching implications of this study are that all jawed vertebrates (including antiarch placoderms) primitively possess both pectoral and pelvic fins and that the pelvic fins did not arise within gnathostomes at a point subsequent to the origin of jaws (Coates, 1994; Janvier, 1996; Goujet, 2001; Zhu et al. 2012). In contrast, these results imply that paired pelvic appendages (pelvic fins/hindlimbs) appeared within gnathostomes before the development of moveable jaws (Zhu et al. 2012).
Fig. 4.
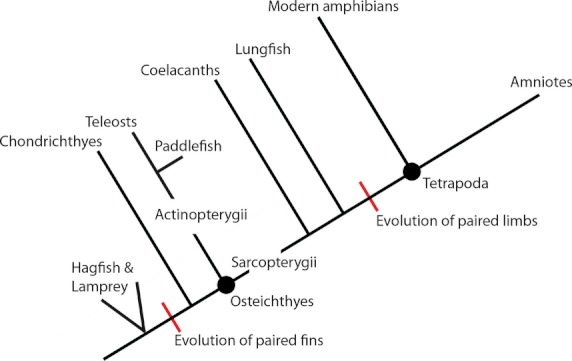
Simplified phylogeny of the living relatives of tetrapods and the evolution of paired appendages based on the results of Inoue et al. (2003).
Pelvic fins and the tetrapod transition
The fish-to-tetrapod transition involved a gradual shift towards more coastal and terrestrial environments (Clack, 2000) and with it came a change in pelvic fin function. This transition involved the shift from paired pectoral and pelvic fins to the development of weight-bearing fore and hindlimbs for locomotion on land. It is thought that the fin to limb transition first began in the pectoral fins and that the evolution of pelvic fins into hindlimbs occurred in a relatively brief period of time between Panderichthys and Acanthostega (Coates, 1996; Boisvert, 2005). Unfortunately, there is a real paucity of fossils in this interval with intact pelvic fins. However, the insights gained from recent fossil finds, re-examination of older fossils and evidence obtained from developmental biology challenge the old ideas and suggest that the pelvic fin to hindlimb transition was evolving even before early tetrapods moved out of the water and colonised land. Two key developmental breakthroughs during this time were the elaboration of the distal skeleton and the development of a robust weight-bearing pelvis (Boisvert, 2005; Johanson et al. 2007).
Evidence from the fossil record and developmental studies of living sarcopterygians suggest that during the evolution of the distal pelvic fin skeleton the digits appeared before the full complement of ankle elements (Wagner & Chiu, 2001; Coates, 2003; Clack & Ahlberg, 2004; Johanson et al. 2007). It is currently thought that digits were not an evolutionary novelty of tetrapods, as previously believed, but evolved from the pre-existing distal radials of sarcopterygians (Johanson et al. 2007; Boisvert et al. 2008). During this stage of evolution, polydactyly was plesiomorphic amongst Tetrapodomorpha. Fossil evidence from early Devonian tetrapods indicates that Ichthyostega had seven toes, Acanthostega had eight toes, and Tulerpeton had at least six toes (Coates & Clack, 1990; Lebedev & Coates, 1995; Coates, 1996). It is thought that pentadactyly of later tetrapods did not evolve until the Carboniferous period (Coates, 1994, 1996).
The evolution of the full complement of the central bones of the ankle (the mesopodium) came after the evolution of digits (Wagner & Chiu, 2001; Coates, 2003; Clack & Ahlberg, 2004; Johanson et al. 2007). The pelvic fins of ancestral sarcopterygians possessed the long bones equivalent to a femur, tibia and fibula, and distal radials from which digits would evolve, but did not possess the full complement of bones of the mesopodium (Andrews & Westoll, 1970; Wagner & Chiu, 2001; Coates et al. 2002; Johanson et al. 2007; Boisvert et al. 2008). Recent re-examination of Panderichthys has revealed that the pelvic fin of this tetrapodomorph fish has a proximal mesopodium element, the fibulare, but lacks the central bones of the mesopodium (Boisvert, 2005). Two of the earliest tetrapods with well preserved hindlimbs, Ichthyostega and Acanthostega, had hindlimbs that had more derived characteristics, but still had very few central bones of the mesopodium (Jarvik, 1980, 1996; Coates, 1996; Johanson et al. 2007). The full complement of the central bones of the ankle seems to appear in Tulerpeton, which has 12 preserved tarsal bones, including three central elements (Lebedev & Coates, 1995). Most Carboniferous tetrapods have three to four central elements in the mesopodium, which allows for the ankle flexibility necessary for walking on land (Coates, 1996).
The development of a robust weight-bearing pelvis was a key step in the evolution of the hindlimb during the tetrapod transition onto land. To walk on land, the relatively gracile unattached pelvic girdle of fish gradually transformed into a large tripartite weight-bearing structure connected to the vertebral column (Fig. 5). The pelvic girdle of lobe-finned fish is composed of a crescentric pubis often connected through cartilage at the midline, but lacks an ilium and is not connected to the vertebral column (Fig. 5A) (Ahlberg, 1989). In contrast, the pelvis of tetrapods has an ilium that is fused to the vertebral column and an ischium that is posterior to the pubis. In addition, the ischium and the pubis from both halves of the pelvis are fused along their midline, which creates a weight-bearing pelvis (Fig. 5B) (Clack, 2000). There is much evidence from both sides of this transition, but little information about how this evolution occurred due to the paucity of fossils from this period with intact pelvic girdles. On one side of the transition, the pelvic girdle of the tetrapodomorph Panderichthys is small, flat, club-shaped and distinctly fish-like (Boisvert, 2005). Unfortunately, the pelvic girdle and fin of the more crownward tetrapodomorph Tiktaalik has not been preserved, but the early tetrapods, Ichthyostega and Acanthostega, had already evolved a distinctively tetrapod-like pelvis with an ilium and ischium (Jarvik, 1980, 1996; Coates, 1996).
Fig. 5.
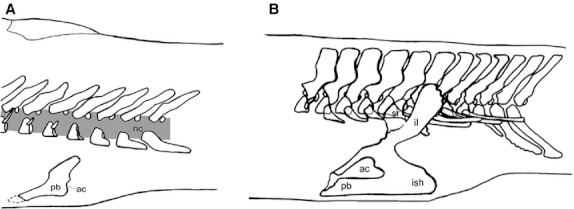
(A) In sarcopterygian fish the pelvic girdle is supported by the hypaxial musculature and consists of a pubis (pb) with a caudally oriented acetabulum (ac) (articulation to the fin) (redrawn from Andrews & Westoll, 1970). (B) In early tetrapods the pelvic girdle consists of a pubis, an ischium (ish), and an ilium (il), which connects to the vertebral column through the sacral rib (sr). The acetabulum is placed laterally (redrawn from Coates, 1996). Figure modified from Cole et al. (2011).
With the evolution of the distal pelvic appendage skeleton and the pelvis, came a shift in locomotory dominance from ‘front wheel drive’ to ‘rear wheel drive’ during the tetrapod transition (Boisvert, 2005). Non-sarcopterygian fish predominately use body muscle undulations and pectoral fins for locomotion, whereas tetrapods use their hindlimbs for this function (Coates et al. 2002). Recent evidence from African lungfish (Protopterus annectens) has shown that this sarcopterygian fish can use a range of pelvic fin-driven gaits such as walking and bounding and use their pelvic fins to lift their body clear of the substrate in an aquatic environment (King et al. 2011). Descriptions of the paired pectoral and pelvic fins of fossils such as Panderichthys and Ichthyostega also offer insights into the evolution of tetrapod locomotion. Panderichthys probably employed an intermediate ‘front-wheel drive’ mode of locomotion, using its pelvic fins as minor anchors while body-flexion propulsion pushed the fish forward (Boisvert, 2005). A recent study of limb joint mobility of Ichthyostega has shown that this early tetrapod had terrestrially ineffectual hindlimbs, as it lacked the necessary rotary motions in its hindlimbs to lift its body off the ground and therefore could not employ lateral sequence walking (Pierce et al. 2012). This new study indicates that early tetrapods went through a stage of hip-joint restriction before they evolved the locomotory behaviours of modern tetrapods (Pierce et al. 2012). Recently, Swartz (2012) described a well preserved fossil specimen of the extinct genus of sarcopterygian fish from the Middle Devonian, Tinirau clackae. Tinirau shares many advanced features with later tetrapodomorphs in the pelvic elements. Tinirau is the earliest known stem tetrapod to have a significantly reduced postaxial process, and a fibula more like those of later tetrapods. Caudally, the pelvis articulates with a femur that is preserved in association with the acetabulum. The postaxial fibular process is highly reduced and displays a similar ‘lip’ overhanging the postaxial edge of the fibulare. The lack of a prominent postaxial process in the fibula of Tinirau is more similar to the condition observed in crownward taxa. This pattern underscores previous phylogenetic reconstructions of the appendicular skeleton in which conventional crown group limb characteristics first originate in the pelvic fins.
Historically, the evolution of the neural control in the pelvic fins and hindlimbs associated with this transition has not received much attention. However, a recent review has compared the organization of the motor neurons in the spinal cord of various vertebrates which aids in the understanding of the evolution of fin/limb motor circuitry necessary for hindlimb dominated locomotion in vertebrates (Murakami & Tanaka, 2011).
In addition to insights gained from recent fossil finds and the re-examination of older fossils, discoveries of preserved pelvic fins and girdles of more crownward transitional Devonian tetrapodomorph fish are eagerly awaited to shed light on the evolution of the vertebrate hindlimb from the pelvic fins of ancestral fish.
Development
Recent examination of the mechanisms involved in the initiation, outgrowth and patterning of hindlimbs among different classes of extant vertebrates have given insights into the evolution of vertebrate hindlimbs. Approaches using model organisms, such as mouse, chick and teleosts, in addition to approaches using extant non-model organisms positioned at strategically important points in the vertebrate phylogeny have shed light upon the important players in hindlimb development. These studies have shown the high degree of conservation in the genetic mechanisms of fin and limb formation between fish and tetrapods but some species-specific differences are present.
Hindlimb development has been mainly investigated in chick and mouse, whereas there have been relatively few studies of pelvic fin development in teleosts and cartilaginous fish. The zebrafish (Danio rerio), a powerful, genetically tractable model organism, has recently been utilised to study pectoral fin/forelimb developmental mechanisms; however, only a few studies have focused on the pelvic fins. Zebrafish pelvic fin buds develop around 3 weeks post-fertilisation as two small mesenchymal bulges that emerge from the lateral plate mesoderm (LPM) of the abdominal flank of the fish (Grandel & Schulte-Merker, 1998). The mesenchymal bulges in the pelvic fin region proliferate to create two pelvic fins bud covered at their distal edge by an apical ectodermal thickening (Grandel & Schulte-Merker, 1998). This structure, which is thought to be analogous to the tetrapod apical ectodermal ridge (AER; Grandel & Schulte-Merker, 1998), is also seen during trout pelvic fin development, where it was termed the pseudoapical ectodermal ridge (Geraudie, 1978). The apical ectodermal thickening of the pelvic fins is only transient and becomes the apical fold which is formed by a layer of dorsal and ventral cylindrical cells (Grandel & Schulte-Merker, 1998). As the apical fold of the pelvic fin is infiltrated by the migrating mesenchyme it morphs into a fin fold, which gives rise to the adult fin (Grandel & Schulte-Merker, 1998).
The early stages of hindlimb development in fish fins are similar to those of tetrapod (mouse and chick) hindlimbs. In the chick embryo, the LPM in the prospective hindlimb region thickens and an AER forms in the overlying ectoderm around 3 days post-fertilisation (reviewed in Saunders, 1977). The hindlimb then continues to grow and elongate. In the mouse embryo, the hindlimb buds first appear around 10 days post-fertilisation, but the AER appears later than in the chick (Martin, 1990). Like the chick, the mouse limb continues to grow and elongate; however, there is a difference in shape between developing chick and mouse hindlimbs.
Positioning
Development of paired appendages at appropriate levels along the primary body axis is a hallmark of the body plan of jawed vertebrates. In all jawed vertebrates, the paired appendages arise from a region known as the lateral competent stripe (Yonei-Tamura et al. 2008). The lateral competent stripe is defined as a region along the flank that is competent for paired appendage development if the correct signals are received (Yonei-Tamura et al. 2008). The entire length of the lateral competent stripe of all jawed vertebrates studied has the competency to form paired appendages (Yonei-Tamura et al. 2008). The utilisation of the lateral stripe differs greatly, with each species having a different ratio for the forelimb/pectoral fin, interlimb and hindlimb/pelvic fin regions. Differences in regulation of the Hox genes in the paraxial mesoderm are thought responsible for the variations in the positioning of paired appendages (Burke et al. 1995; Sordino et al. 1995; Burke & Nowicki, 2003; Noro et al. 2011).
Hox genes are a family of transcriptional regulators that are involved in axial patterning of many structures in vertebrates (Gruss & Kessel, 1991; Krumlauf, 1994; Burke et al. 1995; Deschamps et al. 1999), including fins and limbs (Yokouchi et al. 1991; Sordino et al. 1995; Nelson et al. 1996). In jawed vertebrates, the Hox genes are classified into 13 paralogous groups and are tightly clustered at four loci: HoxA to HoxD. A clear correspondence between particular Hox groups and defined morphological boundaries along the antero-posterior axis of jawed vertebrates has been documented (Gaunt, 1994; Burke et al. 1995; Cohn & Tickle, 1999; Kmita & Duboule, 2003). It has long been known that Hox genes have a unique ability to establish morphologies along the anterior–posterior axis of an embryo by establishing a pre-pattern of the embryonic axis (Lewis, 1978; reviewed Garcia-Fernandez, 2004;). It is also known that positional differences along the body axis, such as the positions of the limbs, are specified by the staggered boundaries of Hox gene expression (Cohn et al. 1997; Akam, 1998; Marshall et al. 1996; Krumlauf, 1994). Noro et al. (2011) showed that the regionalisation of the presomitic mesoderm along the embryonic axis by the Hox code is responsible for the specification of the limb and flank fields in the chick embryo (Noro et al. 2011). Through transplant and ablation experiments it was shown that the presomitic mesoderm adjacent to the prospective limb field is permissive to the development of a limb field and affects the size of the limb field while the paraxial mesoderm adjacent to the flank suppresses limb bud development (Noro et al. 2011). In addition, it has been suggested that Hox transcription factors may be responsible for the restricted patterns of Tbx4 (Minguillon et al. 2005). The position of the pelvic fins/hindlimbs in jawed vertebrates is thought to be determined by Hoxb9, Hoxc9, Hoxd9 and Hoxc10 activity in the developing embryo (Cohn et al. 1997; Lance-Jones et al. 2001; Tanaka et al. 2005; Choe et al. 2006; Murata et al. 2010).
There is a phylogenetic correlation between Hoxd9 expression and hindlimb/pelvic fin positioning. During very early chick development and teleost development, the primary pattern of Hoxb9, Hoxc9 and Hox9d expression is staggered in the prospective forelimb/pectoral fin region, and strong expression is also observed in the interlimb/fin and prospective hindlimb/pelvic fin region (Cohn et al. 1997; Tanaka et al. 2005) (Fig. 6). At later stages of development, during tetrapod limb bud formation, Hoxd9 expression disappears from the interlimb region and is restricted to domains adjacent to the prospective limb buds (Cohn et al. 1997) (Fig. 6A). During pelvic fin budding in the threespine stickleback fish (Gasterosteus aculeatus), the expression domain of Hoxd9 extends from the prospective pelvic region and is maintained in the interlimb region (Tanaka et al. 2005) (Fig. 6B). It is thought that Hoxd9 activity is particularly important for the development of pelvic fins/hindlimbs as its absence is thought to be the cause of the loss of pelvic fins in fugu (Takifugu rubripes), in a mechanism analogous to the loss of pectoral axial patterning cues in python snakes (Pythonidae) (Cohn & Tickle, 1999; Tanaka et al. 2005).
Fig. 6.

In situ hybridisation of Hoxd9 during pelvic fin/hindlimb positioning in chick (A) and threespine stickleback (B). (A) In limb budding stages in chick Hoxd9 expression disappears from the interlimb region and is restricted to domains adjacent to the prospective limb buds (image reproduced with permission from Cohn et al. 1997). (B) During pelvic fin development stages in threespine stickleback, Hoxd9 expression extends from the prospective pelvic region and is maintained in the interlimb region (image reproduced with permissions from Tanaka et al. 2005).
Recent work has also showed a conserved role for Hoxc10 in the positioning of hindlimbs/pelvic fins. In tetrapods, the hindlimb buds arise in the body wall at the level of Hox10 expression in the spinal cord (Lance-Jones et al. 2001; Choe et al. 2006). However, it was observed that in teleosts the level of Hoxc10a expression did not correlate to the position of pelvic fins in the adult fish (Murata et al. 2010). However, Murata et al. (2010) have shown that pelvic fin precursor cells do, in fact, lie next to the posterior expression boundary of Hoxc10a and that these cells migrate to the pelvic fin field due to trunk-tail protrusion of the growing embryo. In addition, it has been shown in knockout mice and zebrafish morphants that removal of the TGF beta family member, Gdf11, changes the expression domain of Hoxc10 and subsequently changes the axial position of the hindlimb/pelvic fin (McPherron et al. 1999; Murata et al. 2010).
Pelvic fin/hindlimb-type specification
An important step during the development of pelvic fins/hindlimbs is the establishment of a fin/limb-type specification. In the past, two genes from the T-box transcription factor family, Tbx4 and Tbx5, were thought to determine limb identity, due to their expression patterns (Gibson-Brown et al. 1996; Tamura et al. 1999; Ruvinsky et al. 2000). In amniotes, teleosts and anurans Tbx4 is expressed in developing hindlimbs and Tbx5 is expressed in developing forelimbs (Gibson-Brown et al. 1996; Logan et al. 1998; Tamura et al. 1999; Ruvinsky et al. 2000; Takabatake et al. 2000) (Fig. 7). Historically, this expression pattern was thought to be conserved across vertebrates with paired limbs.
Fig. 7.
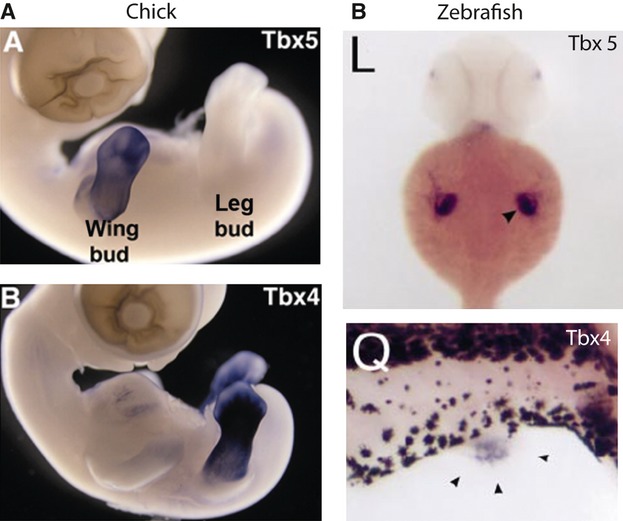
In situ hybridisation of Tbx4 and Tbx5 in chick (A) and zebrafish (B). (A) Tbx5 is expressed in the developing wing buds of chick embryos; Tbx4 is expressed in the developing leg buds (figure reproduced with permission from Logan, 2003). (B) In zebrafish, Tbx5 is expressed in the developing pectoral fins and Tbx4 is expressed in the developing pelvic fins (figure reproduced with permission from Ruvinsky et al. 2000).
However, there is conflicting evidence about the ability of Tbx4 and Tbx5 to confer limb-type identity. Ectopic gene misexpression studies in chick embryos have shown that Tbx4 and Tbx5 play a role in determining limb-type morphology. Ectopic expression of Tbx5 in the developing chick hindlimb bud can confer forelimb-type morphology to the developing limb and induce ectopic expression of forelimb markers (Rodriguez-Esteban et al. 1999; Takeuchi et al. 2003). Equally, it has been shown that misexpression of Tbx4 in developing chick forelimb buds gives the developing limb hindlimb-type morphology and induces ectopic expression of hindlimb markers (Takeuchi et al. 2003). However, this is in direct contrast to some results obtained from other species as detailed below.
In mice, gene deletion-gene replacement studies using knockout and transgenic mice have shown that in the absence Tbx5 in the forelimb, Tbx4 does not confer hindlimb-like morphology to the limb (Minguillon et al. 2005, 2009). If Tbx5 is ablated in the forelimb of mice and replaced with ectopic Tbx4, the forelimbs have a normal forelimb-type pattern of gene expression and a forelimb-type skeletal morphology (Minguillon et al. 2005). These results suggested that other factors must determine limb-type morphology. In addition, it has been shown that there is a low level of expression of Tbx4 in the developing forelimbs of mouse (Gibson-Brown et al. 1996; Naiche et al. 2011). The forelimb expression domain of Tbx4 has been shown to be unnecessary for forelimb morphology in mice and the enhancer that drives this expression is not evolutionarily conserved outside of the mammalian class (Menke et al. 2008; Naiche et al. 2011). However, this is similar to the situation in urodeles, where both Tbx4 and Tbx5 are expressed in the developing fore and hindlimbs of N. viridescens during limb development (Khan et al. 2002). These results shed doubt on the specific role of Tbx4 in determining hindlimb-like identity. In contrast, studies by Ouimette et al. (2008) showed that while Tbx4 does not confer hindlimb-like morphology when ectopically expressed in the forelimb, it is sufficient to rescue hindlimb-like morphology to Pitx1 null hindlimbs, which lack hindlimb-like morphology. Ouimette et al. (2008) also demonstrated that Tbx4 has a unique transcriptional repressor site which is possibly responsible for hindlimb-like morphology, and that this transcriptional repressor site is absent in Tbx5 and the ancestral Tbx4/5 cluster. It is important to note that due to the coupling of limb initiation and limb-type morphology the rescued hindlimb-like morphologies may be due to the activity of Tbx4 on hindlimb-like morphology or the return of the normal hindlimb development initiation cascade.
Although the studies on Tbx4 and Tbx5 appear to be inconclusive concerning their role in limb-type specification, it is clear that there are other factors involved in this process. The paired-like homeodomain transcription factor, Pitx1, is expressed in the region forming the pelvic appendage and in the pelvic appendage buds, but not in the forelimb regions (Lamonerie et al. 1996; Cole et al. 2003). Pitx1 is thought to be involved in both hindlimb identity specification and hindlimb development (Lamonerie et al. 1996; Lanctot et al. 1999). In tetrapods, expression of Pitx1 is observed before the onset of Tbx4 expression (Lamonerie et al. 1996). In both mouse and chick, misexpression of Pitx1 in the forelimb imparts hindlimb-like characteristics to the muscles, skeleton and skin (Logan & Tabin, 1999; Takeuchi et al. 1999; DeLaurier et al. 2006). In addition, Pitx1 null mice develop hindlimbs in which the skeleton has lost some of its hindlimb-like characteristics (Lanctot et al. 1999; Szeto et al. 1999; Marcil et al. 2003). However, as Pitx1 null mice do develop hindlimbs and not all hindlimb-like features are affected, it is possible that other factors are involved in the specification of hindlimb-type morphologies in tetrapods (Fig. 8). The role of Pitx1 in specifying pelvic fin identity still needs to be determined in fish to confirm whether its hindlimb identity role is conserved amongst vertebrates.
Fig. 8.
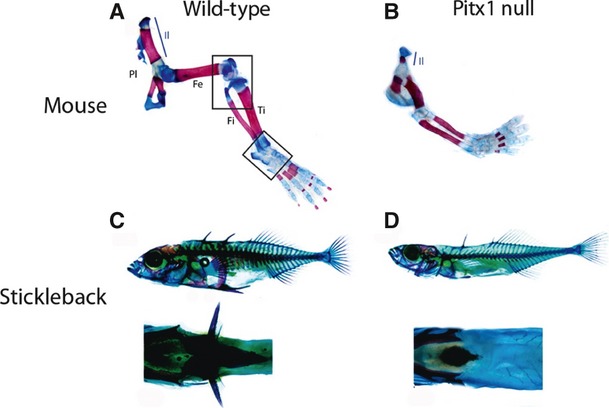
Pelvic appendage skeletal preparations from wild-type (A) and Pitx1 null (B) mice and wild-type (C) and Pitx1 null threespine stickleback (D). (A, B) Mice lacking Pitx1 develop small hindlimbs that have lost some hindlimb characteristics (figure reproduced with permission from Duboc & Logan, 2011). (C, D) Threespine stickleback which lack Pitx1 in the pelvic regions do not develop pelvic spines (figure reproduced with permission from Cole et al. 2003).
Initiation
Several extracellular signalling molecules and transcription factors have been reported to be involved in initiating hindlimb bud development in chick and mouse. More recently, a few of these initiation factors have been investigated in teleosts, showing that many of the same molecules seem to be employed (Gibson-Brown et al. 1996; Tamura et al. 1999; Ruvinsky et al. 2000; Cole et al. 2003; Shapiro et al. 2004). The origin of the limb/fin bud induction signal remains controversial and there is evidence to show that it begins in either the presomitic/paraxial mesoderm or the intermediate mesoderm. Historic studies on chick forelimb bud induction have shown that a foil barrier placed between the somites and the LPM blocks forelimb bud induction (Murillo-Ferrol, 1965; Sweeney & Watterson, 1969; Stephens & McNulty, 1981). However, these results have been reinterpreted by Crossley et al. (1996) as blocking an Fgf8 signal from the intermediate mesoderm, which subsequently caused a failure of chick limb bud induction. More recent work in zebrafish fins has shown that the fin bud induction signal begins in the paraxial mesoderm and is transferred by secondary signals to the LPM and the overlying ectoderm (Begemann et al. 2001; Grandel et al. 2002; Gibert et al. 2006; Mercader et al. 2006; Grandel & Brand, 2011). As the paraxial mesoderm and the LPM are separated by the intermediate mesoderm, it seems likely that molecules from the paraxial mesoderm could signal to the LPM through the intermediate mesoderm, where both the paraxial mesoderm and the intermediate mesoderm would have a role in limb/fin bud initiation.
The role of Fgf signalling and the intermediate mesoderm in pelvic fin/hindlimb bud induction
Previous work in tetrapods has suggested that Fgf activity in the intermediate mesoderm might trigger the induction of limb budding by transferring a signal to the limb fields of the LPM (Crossley et al. 1996; Vogel et al. 1996). The Fgf8 gene product was thought to play this role, as it is expressed in the intermediate mesoderm adjacent to the limb-forming regions before and during limb bud induction and can maintain cells in a proliferative state (Crossley et al. 1996; Vogel et al. 1996). Furthermore, it has been shown that the application of an Fgf8-soaked bead to the interlimb region of tetrapods can induce an ectopic limb to form (Cohn et al. 1995; Mahmood et al. 1995; Ohuchi et al. 1995; Crossley et al. 1996; Vogel et al. 1996; Yonei-Tamura et al. 1999).
However, recent studies have demonstrated that conditional removal of certain Fgf activity from the mesoderm has little effect on limb bud induction in mice (Boulet et al. 2004; Perantoni et al. 2005). When Fgf8 activity is transgenically removed from the mesoderm of mice, the mutant's limbs form at the normal position and time (Perantoni et al. 2005). Likewise, transgenic mice lacking both Fgf4 and Fgf8 expression in the mesoderm also develop limbs which form at the normal position and time (Boulet et al. 2004). Additionally, in zebrafish, it has been shown that Fgf signalling is not required for pectoral fin bud initiation (Mercader et al. 2006). Zebrafish embryos treated with the Fgf receptor antagonist, SU5402, display normal pectoral fin bud induction in the face of a lack of Fgf signalling (Mercader et al. 2006). Despite controversy over a role of Fgf activity during limb/fin bud induction, Fgf activity is certainly necessary during later stages of limb/fin budding and development.
The role of retinoic acid and the somites in pelvic fin/hindlimb bud induction
Recently, it has been proposed that a retinoic acid-controlled signal is responsible the onset of Tbx5 expression in pectoral fins and the induction of pectoral fin buds (Gibert et al. 2006; Mercader et al. 2006; Grandel & Brand, 2011). Evidence from these studies suggests that retinoic acid and Tbx5 signalling is upstream of Fgf function which is recently thought to play only a local role in the limb bud during induction, at least in zebrafish (Gibert et al. 2006; Mercader et al. 2006; Grandel & Brand, 2011).
In zebrafish, studies of retinoic acid signalling have shown that its effect on pectoral fin bud induction is probably indirect and likely to be mediated by secondary signals (Mercader et al. 2006). It has been shown that wnt2b relays the retinoic acid fin bud induction signal to the pectoral fin field and induces Tbx5 expression (Mercader et al. 2006). In chick embryos, wnt2b in forelimbs and wnt8c (previously known as wnt8a) in hindlimbs are thought be involved in limb bud induction (Kawakami et al. 2001). In the chick embryo, wnt8c is expressed in a domain that suggests it may be involved in hindlimb bud induction (Kawakami et al. 2001). It addition, ectopic expression of wnt8c has been shown to induce hindlimb buds along the flank of chick embryos (Kawakami et al. 2001).
However, neither wnt2b nor wnt8c have been detected in the limb buds of early mice embryos (Agarwal et al. 2003). This may be due to the fact that Tbx5 is not necessary for the establishment of the limb field in this species, as it is in zebrafish, or that the limb bud induction signal may come from the intermediate mesoderm and not the somitic mesoderm in mice (Crossley et al. 1996; Agarwal et al. 2003; Ahn et al. 2002; Gibert et al. 2006; Mercader et al. 2006; Grandel & Brand, 2011). Recent studies from mice have confirmed that although retinoic acid promotes mouse forelimb bud induction, it is not necessary for hindlimb bud induction, as retinoic acid-deficient mice develop normal hindlimb buds (Zhao et al. 2009). These authors concluded that the role of retinoic acid in mouse limb development was to suppress Fgf8 activity along the flank, allowing for a permissive domain for forelimb bud induction to occur; however, this mechanism is not necessary for Fgf8 regulation at the time the hindlimbs develop (Zhao et al. 2009). The difference between permissive signals for fore and hindlimb induction may account for the ability of fish to develop pelvic fins at such a relatively late stage compared to pectoral fins. To date, it remains uncertain which mechanisms lead to the induction of hindlimb/pelvic fin buds.
Downstream pelvic fin/hindlimb bud induction cascade
Although the specific mechanisms of the pelvic fin/hindlimb bud induction cascade remain unknown, secondary signals are thought to trigger the expression of Tbx4 in pelvic fins/hindlimbs. Recent studies in mice have suggested that Tbx4 and Tbx5 play a shared role in limb initiation (Minguillon et al. 2005, 2009). Tbx4 is one of the first genes known to be expressed in the prospective pelvic fin/hindlimb region during pelvic fin/hindlimb bud induction (Gibson-Brown et al. 1996; Tamura et al. 1999; Ruvinsky et al. 2000). In jawed vertebrates, Tbx4 is expressed in the developing pelvic fin/hindlimb just before and during the pelvic fin/hindlimb bud induction stages (Gibson-Brown et al. 1996; Tamura et al. 1999; Ruvinsky et al. 2000). Tbx4 and its forelimb counterpart Tbx5 have been shown to share a transcriptional activator site which has been suggested to be responsible for the shared limb initiation activity of these genes (Ouimette et al. 2008). Under normal conditions, Tbx4 activity leads to the initiation of Fgf10 signalling in the bud mesoderm and the subsequent establishment of the FGF positive signalling feedback loop between the bud mesenchyme and the overlying ectoderm (see later) (Fig. 9). Mouse and chick studies have found that Tbx4 is necessary for proper hindlimb bud induction and subsequent hindlimb outgrowth (Naiche & Papaioannou, 2003, 2007; Takeuchi et al. 2003). Conditional mice knockout models have shown that a loss of Tbx4 activity results in a drastically reduced hindlimb bud which fails to complete limb bud induction, while overexpression of a dominant negative form of Tbx4 in the prospective hindlimb fields of chick embryos results in a complete legless phenotype (Naiche & Papaioannou, 2003, 2007; Takeuchi et al. 2003).
Fig. 9.
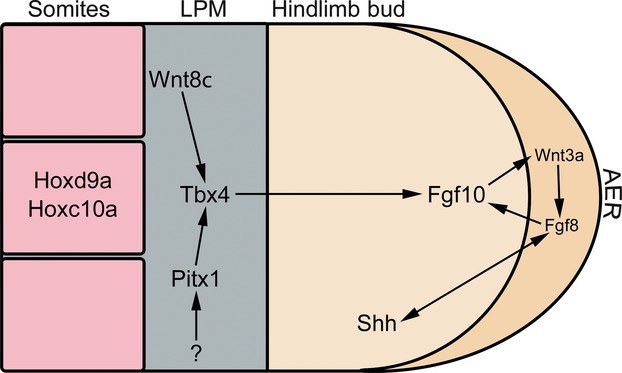
A simplified version of some of the key genes involved in pelvic fin/hindlimb development. It should be noted that some of these genes have not yet been investigated in pelvic fins. Both Hoxd9a and Hoxc10a have been shown to be involved in the positioning of the pelvic appendage in amniotes and teleosts. Wnt8c has the ability to induce ectopic hindlimbs in chick. The hindlimb identity genes Tbx4 and Pitx1 have both been shown to play roles in hindlimb specification and initiation. A positive feedback loop between Fgf10 in the mesoderm, Fgf8 in the apical ectoderm and Shh in the ZPA controls the outgrowth and patterning of the pelvic appendage.
In addition to Tbx4 signalling, it is possible that the hindlimb-related gene Pitx1 may play an important role in hindlimb/pelvic fin bud induction. In tetrapods, Pitx1 is neither necessary nor sufficient for hindlimb bud induction, as Pitx1 null mice possess small hindlimbs and Pitx1 misexpression cannot induce ectopic hindlimbs (Lanctot et al. 1999; Szeto et al. 1999; Minguillon et al. 2005)(Fig. 8A and B). However, Pitx1 is known to partially regulate Tbx4 in developing hindlimb buds, as Pitx1 knockout mice display reduced Tbx4 expression (Lamonerie et al. 1996; Lanctot et al. 1999). Pitx1 is thought to regulate Tbx4 via a Tbx4 upstream enhancer region in tetrapods (Menke et al. 2008). Tetrapods have two Tbx4 enhancer regions, 5′ HLEA and 3′ HLEB, and only the 3′ HELB is conserved in fish (Menke et al. 2008). Only the 5′ HLEA has Pitx1 putative binding sites and it is thought that in tetrapods, Pitx1 regulates Tbx4 expression through this enhancer region (Menke et al. 2008). It remains to be determined whether Pitx1 regulates Tbx4 expression in fish, as it is unknown if the 3′ HLEB enhancer region interacts with Pitx1.
In contrast to mouse hindlimbs, pelvic fin bud induction in teleosts seems to be dependent on Pitx1 activity. It has been shown that the absence of Pitx1 activity in the prospective pelvic regions of certain populations of threespine sticklebacks is linked to the failure of pelvic spine formation at an early stage (Cole et al. 2003; Shapiro et al. 2004) (Fig. 8C and D). The absence of Pitx1 activity and subsequent failure of pelvic spine development has been attributed to regulatory mutations deleting a tissue-specific enhancer of Pitx1, which causes specific pelvic region loss of Pitx1 expression in certain populations of threespine sticklebacks (Chan et al. 2010). Due to the differences in morphology that loss of Pitx1 in tetrapods and teleost causes, it seems possible that Pitx1 plays a slightly different role in teleost pelvic fin development than it does in amniote hindlimb development. It would be of great interest to examine the role of Pitx1 in the induction of hindlimbs in other vertebrates situated phylogenetically between teleosts and amniotes.
Outgrowth
The growth from a bulge in the body wall to a bud that grows out independently is a critical step in limb development and involves interaction between the three main signalling centres; the AER, the zone of polarising activity (ZPA) and the non-ridge ectoderm. These signalling centres are conserved in jawed vertebrates, are interdependent and are established early through communication between the limb/fin bud mesoderm and the overlying ectoderm.
The establishment of the apical ectodermal ridge
The establishment of an AER is essential to the development of amniote hindlimbs and teleost pelvic fins. The AER is a thickened layer of ectodermal cells at the distal tip of a developing limb/fin bud (Fig. 10). In the forelimb/pectoral fin, specific gene expression in the dorsal and ventral ectoderm is thought to induce the distal limb ectoderm to become ridge-like (see Fernandez-Teran & Ros, 2008 for a detailed review; Hatta et al. 1991; Norton et al. 2005). It remains unknown whether a similar process occurs in hindlimbs and pelvic fins and additional experiments are needed. However, in hindlimbs it is known that as the developing hindlimb bud reaches a sufficient size, Tbx4 signalling triggers Fgf10 expression in the mesenchyme of the bud, which in turn is necessary to activate Fgf8 expression in the overlying ectoderm (see Fernandez-Teran & Ros, 2008 for a detailed review). In developing amniote hindlimb buds, mesenchymal Fgf10 signals to Fgf8 in the overlying ectoderm via wnt3a (chick) or wnt3 (mouse) (Barrow et al. 2003; Narita et al. 2005, 2007) (Fig. 9). In mouse, the ongoing activity of Fgf8 in the AER is necessary for proper outgrowth of the hindlimb (Lewandoski et al. 2000; Moon & Capecchi, 2000) (Fig. 11A).
Fig. 10.
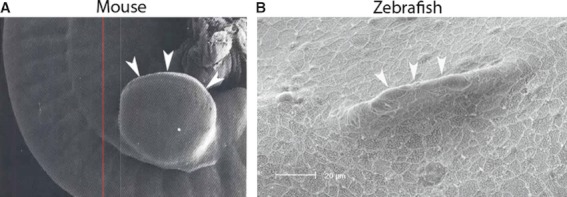
Scanning electron microscopy of the AER of a developing mouse hindlimb bud (A) and a developing zebrafish pelvic fin (B) (figure reproduced with permission from Martin, 1990 and Don et al. 2011).
Fig. 11.
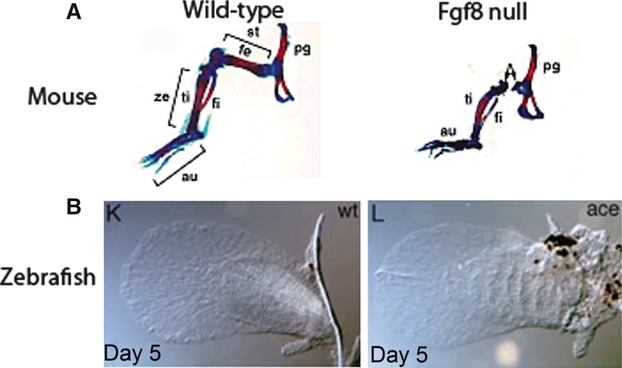
Skeletal preparations of hindlimbs of wild-type and Fgf8 null mice (A) and wild-type and Fgf8 mutant zebrafish pectoral fins (B). While the hindlimbs of Fgf8 null mice have skeletal outgrowth defects, the pectoral fins of Fgf8 mutant zebrafish develop normally (figure reproduced with permission from Lewandoski et al. 2000 and Reifers et al. 1998).
Based on evidence from zebrafish pectoral fin development, it is thought that similar Fgf signalling establishes the pseudoapical ectodermal ridge in teleost pelvic fins. However, in contrast to mouse and chick, in the developing zebrafish pectoral fin buds the expression of Fgf8 is not observed until after the establishment of the AER (Fischer et al. 2003). In fact, Fgf8 is not absolutely necessary for pectoral fin development, as the Fgf8 mutant zebrafish acerebellar develops normal pectoral fins (Reifers et al. 1998) (Fig. 11B). Instead, in developing zebrafish pectoral fin buds, expression of Fgf16 and Fgf24 is seen before the expression of Fgf8 (Fischer et al. 2003; Nomura et al. 2006). In addition, Fgf8 expression is dependent on Fgf16 activity (Fischer et al. 2003; Nomura et al. 2006). The zebrafish mutant, ikarus, which has a point mutation in the coding region of Fgf24, fails to develop pectoral fins which demonstrate that Fgf24 is necessary for proper pectoral fin outgrowth. However, Fgf24 is not necessary for pelvic fin development, as ikarus mutants develop normal pelvic fins (Fischer et al. 2003).
AER maintenance
Despite the differences in morphology, studies of teleosts, amphibians and amniotes have shown that the formation and maintenance of an AER/pseudoapical ectodermal ridge/apical thickening is necessary for proper hindlimb outgrowth and patterning along the proximal distal axis. As the limb bud grows outward, the ridge maintains a zone of undifferentiated mesenchymal cells at the distal tip of the bud, whereas the more proximal cells differentiate. The role of the ridge in promoting limb bud outgrowth was first demonstrated in chick embryos. It was shown that when the ridge is cut away, limb bud outgrowth ceases and a truncated limb develops (Saunders, 1948). Unlike teleosts and amniotes, direct developing frogs, such as the treefrog (Eleutherodactylus coqui), lack a morphological AER, but do have an apical ectodermal thickening that is necessary from proper limb patterning, as its removal results in anterior defects in skeletal patterning (Richardson et al. 1998). However, the defects seen are not as severe as those seen in amniotes, which may be due to the ability of the apical thickening of amphibians to regenerate (Tschumi, 1957; Richardson et al. 1998; Stopper & Wagner, 2005).
The AER and the ZPA
While AER maintenance requires continuous positive Wnt and Fgf signalling between the mesoderm and the overlying ectoderm, another feedback loop involving the ZPA is also necessary for proper AER maintenance and outgrowth. The ZPA is a group of cells located in the posterior mesenchyme of the developing hindlimb bud that express Shh (Saunders & Gasseling, 1968; Riddle et al. 1993). This domain of Shh is conserved between the developing hindlimb/pelvic fin buds of many jawed vertebrates, including mouse, chick and zebrafish (Echelard et al. 1993; Krauss et al. 1993; Roelink et al. 1994; Don EK & Cole NJ, unpublished). However, the evolutionary origin of the ZPA remains unclear, as studies by Tanaka et al. (2002), Dahn et al. (2007) and Yonei-Tamura et al. (2008) have shown key spatial and temporal differences in Shh expression in cartilaginous fish from those found in tetrapods and teleosts. In cartilaginous fish (Chondrichthyes), Shh expression is only seen during later stages of post-budding pelvic fin development and the expression domain does not map to the ZPA of the developing pelvic fins (Tanaka et al. 2002; Dahn et al. 2007; Yonei-Tamura et al. 2008).
Development of the muscles of the pelvic fins/hindlimbs
Recently, the development and evolution of the musculature of the pelvic fin/hindlimb has been examined in several species occupying key phylogenetic positions (Cole et al. 2011). In amniotes, amphibians and the pectoral fins of bony fish, the limb and pectoral fin muscles are generated by limb myoblasts which are derived from the migration of mesenchymal precursor cells (Nicolas et al. 1998; Neyt et al. 2000; Satoh et al. 2005; Vasyutina & Birchmeier, 2006; Sabo et al. 2009). These cells, under the direction of Lbx de-laminate from the hypaxial region of the hindlimb-level somites and migrate to the developing hindlimb mesenchyme (Nicolas et al. 1998; Satoh et al. 2005; Vasyutina & Birchmeier, 2006; Sabo et al. 2009) (Fig. 12A).
Fig. 12.
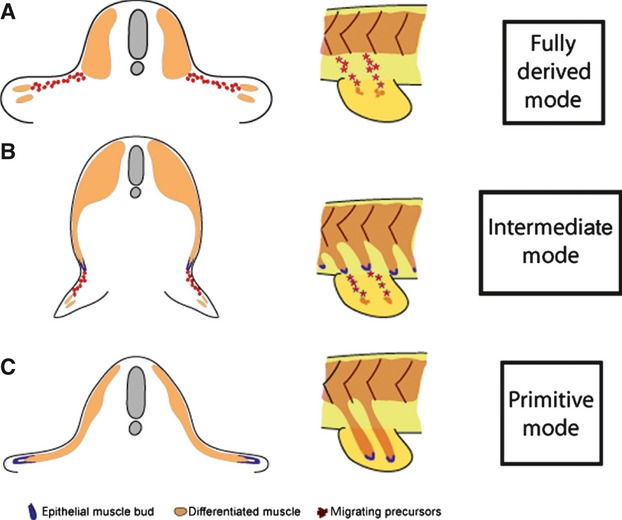
Diagram of the different modes of fin/limb muscle formation. (A) Amniotes and anurans use long-range migration of individual mesenchymal migratory myoblasts in both the fore- and hindlimbs for limb muscle formation. Bony fish deploy this mode of fin muscle formation in the pectoral fins. (B) Zebrafish utilise the long-range migration of individual mesenchymal migratory myoblasts from an epithelial myotomal extension to make the muscle of the pelvic fin. (C) Chondrichthyans utilise the primitive mechanism of direct epithelial extension to generate the muscle of pectoral and pelvic fins (figure reproduced from Cole et al. 2011).
In contrast, within chondrichthyan species, the pectoral and pelvic fin muscles are formed by a migrating epithelial bud tipping a direct myotome extension (Neyt et al. 2000 and references within). The migrating epithelial buds extend ventrally from the myotome and enter the developing fin mesenchyme to generate the paired fin muscles. This mechanism is not under the control of Lbx as no expression of Lbx is seen during this process (Neyt et al. 2000; Cole et al. 2011) (Fig. 12C).
A distinct process which incorporates both primitive and derived characteristics of vertebrate appendicular muscle formation has been described in the pelvic fins of bony fish (Cole et al. 2011). A recent study by Cole et al. (2011) showed that in zebrafish, paddlefish and lungfish pelvic fins, an epithelial myotomal extension tipped by an epithelial bud, extends towards the pelvic fin bud but fails to enter the developing mesenchyme of the pelvic fin bud. Instead, the pelvic fin muscle precursors are carried ventrally by the myotome extension and, once in position, undergo an epithelial–mesenchymal transition. These cells are then induced to express Lbx and migrate into the fin mesenchyme to form the individual pelvic fin muscles (Cole et al. 2011) (Fig. 12B). This mode of muscle formation represents an important step in the evolution of the tetrapod hindlimb muscle developmental mechanisms which evolved during the osteichthyan radiation. By adopting a more derived mode of pelvic fin muscle formation, the pelvic fin could be located anywhere in the dorsoventral body axis, as well as allowing for earlier deployment of muscle precursors to the pelvic fin environment, both of which may have facilitated the development of more robust, weight-bearing hindlimbs.
The later stages of development of muscles of the hindlimbs of tetrapods are thought to be under the influence of Pitx1 and Tbx4. If Tbx4 activity is absent during later stages of hindlimb development, there is no effect on the outgrowth of the limb skeleton but muscle patterning is affected (Hasson et al. 2010). Although, an absence of Tbx4 expression affects hindlimb muscle patterning, misexpression of Tbx4 in the developing forelimb does not confer hindlimb-like morphologies to the muscles of the forelimb (DeLaurier et al. 2006). It seems that Pitx1 may play a greater role in determining hindlimb-like muscle morphology, as misexpression of Pitx1 in the forelimb of mice gives the muscles more hindlimb-like morphologies (DeLaurier et al. 2006). The mechanisms of the later stages of development of the muscles of the pelvic fins are yet to be investigated fully.
Pelvic fin/hindlimb loss
Hindlimbs and pelvic fins show a wide range of morphologies from the legs of man to the modified pelvic fins (claspers) of sharks. In addition, secondary loss of hindlimbs/pelvic fins has occurred repeatedly throughout the evolution of many different lineages, including mammals, reptiles and fish. In some species, hindlimbs are completely absent (Fugu), whereas in others, remnants remain (pythons, whales) (Cohn & Tickle, 1999; Bejder & Hall, 2002; Santini & Tyler, 2003; Tanaka et al. 2005). Further to this, an asymmetry of pelvic fin loss is observed in other species, such as stickleback and manatees (Bell & Orti, 1994; Cole et al. 2003; Shapiro et al. 2004; Shapiro et al. 2006).
Pelvic fin loss is documented in one or more species of 92 teleostean families; the pelvic fin has been lost about 50 independent times, excluding multiple losses within families (Nelson, 1993). Many freshwater populations of threespine sticklebacks have undergone pelvic spine (a modified pelvic fin) loss, ranging from complete loss to asymmetric loss, from an anadromous ancestor (Bell & Orti, 1994; Cole et al. 2003; Shapiro et al. 2004). The genetic basis of pelvic spine loss has been examined in freshwater Scottish and freshwater Canadian populations of threespine sticklebacks and has been determined to be caused by an upstream pelvic region regulator of Pitx1 (Chan et al. 2010). Pelvic fin loss in threespine sticklebacks was also shown to be asymmetric, with greater reduction of the right than the left (Cole et al. 2003; Shapiro et al. 2004). This right–left asymmetry has been attributed to the partial functional compensation of a closely related gene Pitx2, which is preferentially expressed on the left side during development (Shapiro et al. 2004). However, no Pitx2 transcripts were detected in the pelvic regions of pelvic spine-deficient threespine sticklebacks during pelvic spine development stages (Cole et al. 2003). The same right–left asymmetry is also seen in the hindlimbs of Pitx1 knockout mice and it has been suggested that the right–left asymmetry seen in the hindlimb remnants of manatees may also be due to the same Pitx1/Pitx2 mechanism (Lanctot et al. 1999; Marcil et al. 2003; Minguillon et al. 2005; Szeto et al. 1999; Shapiro et al. 2006).
In teleosts, different mechanisms account for pelvic fin loss in different species. Adult pufferfish completely lack a bony pelvic apparatus and display no evidence of pelvic fin bud development (Santini & Tyler, 2003; Tanaka et al. 2005). The cause of pelvic fin loss in this species is thought to be caused by an absence of late onset Hox gene expression, specifically Hoxd9a (Tanaka et al. 2005). Some populations of zebrafish have also undergone secondary hindlimb loss. Pelvic finless zebrafish initiate pelvic fin development and form mesenchymal bulges in the pelvic region but fail to form a morphological pseudoapical ectodermal ridge (Don et al. 2011). The pelvic fin buds then regress and no pelvic fins or pelvic skeletal apparatus is present in the adult fish (Don et al. 2011).
Secondary loss of hindlimbs has also occurred in many tetrapod lineages, such as snakes, lizards and cetaceans. All limbless tetrapods are descended from limbed ancestors. Extant snakes have evolved from limbed ancestors, as demonstrated by extinct snakes with hindlimbs, such as Pachyrhachis problematicus, Haasiophis terrasanctus and Podophis descouensi (Haas, 1980; Rieppel, 1988; Lee & Caldwell, 1998; Coates & Ruta, 2000; Greene & Cundall, 2000; Rage & Escuillié, 2000; Tchernov et al. 2000). In more primitive snakes, such as pythons, a rudiment of a hindlimb bud forms in the embryo (Cohn & Tickle, 1999). The early hindlimb bud is initiated and begins to develop, but fails to continue outgrowth and a developed AER and ZPA are never formed (Cohn & Tickle, 1999). After a series of experiments demonstrating that the mesoderm of developing python hindlimbs is capable of inducing both an AER and a ZPA in chick limb buds, it was suggested that the cause of hindlimb loss in pythons is due to changes in mesodermal Hox gene expression (Cohn & Tickle, 1999). Whereas primitive snakes initiate hindlimb development, more advanced snakes completely lack hindlimb buds (Cohn & Tickle, 1999). Similar to hindlimb loss in primitive snakes, many limbless lizards also initiate hindlimb development (Camp, 1923; Essex, 1927; Gans, 1975; Presch, 1975). However, unlike primitive snakes, the hindlimb buds of slow worms and green lizards do develop an AER, which then regresses (Raynaud et al. 1974; Raynaud, 1990).
Although all modern cetaceans lack external hindlimbs, vestigial elements of the hindlimb skeleton can be found in many adult cetaceans (Andrews, 1921; Howell, 1970). This is possible because the embryos of modern cetaceans form rudimentary hindlimb buds (Sedmera et al. 1997a,b; Bejder & Hall, 2002 and references therein). The hindlimb buds of whales develop an AER and progress to the condensation stage, where vascular plexuses outline the condensations for the digits and the nerves grow into the developing hindlimb buds (Sedmera et al. 1997a; Bejder & Hall, 2002). There is a correlation between the time the hindlimb buds persist and the elements of the hindlimb skeleton that remain (Andrews, 1921; Howell, 1970; Bejder & Hall, 2002). To date, the genetic mechanism responsible for hindlimb loss in whales is unknown. Early studies of developing spotted dolphin described the hindlimb buds as having an underdeveloped AER; however, more recent studies suggest otherwise (Sedmera et al. 1997a; Thewissen et al. 2006). Recent data suggests that an AER is established in the hindlimb buds of spotted dolphin embryos; however, neither this structure nor Fgf8 expression is maintained (Thewissen et al. 2006). In addition, Shh expression is absent from the developing hindlimb buds and a ZPA is not formed, which is thought to be due to the absence of Hand2, an upstream regulator of Shh (Thewissen et al. 2006). It appears that many different genetic mechanisms are responsible for secondary hindlimb loss. The discovery of the mechanisms responsible for these cases of hindlimb loss could yield many exciting new insights into the evolution of hindlimb loss and hindlimb development in general.
Conclusions
Fascinating new insights into the field of pelvic fin/hindlimb developmental evolution have shed light on both developmental and evolutionary mechanisms. Studies of the pelvic fins/hindlimbs of model and non-model organisms in higher and lower vertebrates have shown that hindlimb developmental mechanisms are conserved throughout evolution with species specific differences accounting for the varied morphologies and functions observed. We now know more than ever before about the evolution of hindlimb developmental mechanisms and are at a point that we can readily compare organisms.
Acknowledgments
We would like to acknowledge Dr Zerina Johanson, Dr Greg Edgecombe and Claire Winnick for revision of the manuscript and also two anonymous reviews whose comments significantly improved this manuscript.
References
- Agarwal P, Wylie JN, Galceran J, et al. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- Ahlberg PE. Paired fin skeletons and relationships of the fossil group Porolepidormes (Osteichthyes: Sarcopterygii) Zool J Linn Soc. 1989;96:119–166. [Google Scholar]
- Ahlberg PE, Milner AR. The origin and early diversification of tetrapods. Nature. 1994;368:507–514. [Google Scholar]
- Ahlberg PE, Clack JA, Blom H. The axial skeleton of the Devonian tetrapod Ichthyostega. Nature. 2005;437:137–140. doi: 10.1038/nature03893. [DOI] [PubMed] [Google Scholar]
- Ahlberg PE, Clack JA, Luksevics E, et al. Ventastega curonica and the origin of tetrapod morphology. Nature. 2008;453:1199–1204. doi: 10.1038/nature06991. [DOI] [PubMed] [Google Scholar]
- Ahn D, Kourakis MJ, Rohde LA, et al. T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature. 2002;417:754–758. doi: 10.1038/nature00814. [DOI] [PubMed] [Google Scholar]
- Akam M. Hox genes, homeosis and the evolution of segment identity: no need for hopeless monsters. Int J Dev Biol. 1998;42:445–451. [PubMed] [Google Scholar]
- Andrews RC. A remarkable case of external hind limb in a humpback whale. Am Mus Novitates. 1921;9:1–9. [Google Scholar]
- Andrews SM, Westoll TS. The postcranial skeleton of Eusthenopteron foordi Whiteaves. Trans R Soc Edinburgh. 1970;68:207–328. [Google Scholar]
- Balfour FM. On the development of the skeleton of the paired fins of Elasmobranchii, considered in relation to its bearings on the nature of the limbs of the vertebrata. Proc Zool Soc Lond. 1881;1881:656–671. [Google Scholar]
- Barrow JR, Thomas KR, Boussadia-Zahui O, et al. Ectodermal Wnt3/β-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch G, Jr, et al. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Bejder L, Hall BK. Limbs in whales and limblessness in other vertebrates: mechanisms of evolutionary and developmental transformation and loss. Evol Dev. 2002;4:445–458. doi: 10.1046/j.1525-142x.2002.02033.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Orti G. Pelvic reduction in threespine stickleback from Cook Inlet Lakes: geographical distribution and intrapopulation variation. Copeia. 1994;1994:314–325. [Google Scholar]
- Boisvert CA. The pelvic fin and girdle of Panderichthys and the origin of tetrapod locomotion. Nature. 2005;438:1145–1147. doi: 10.1038/nature04119. [DOI] [PubMed] [Google Scholar]
- Boisvert CA, Mark-Kurik E, Ahlberg PE. The pectoral fin of Panderichthys and the origin of digits. Nature. 2008;456:636–638. doi: 10.1038/nature07339. [DOI] [PubMed] [Google Scholar]
- Boisvert CA, Ahlberg PE, Joss J. Comparative Pelvic Development of the Axolotl (Ambystoma mexicanum) and the Australian Lungfish (Neoceratodus Forsteri. In Boisvert, Catherine Anne. The Origin of Tetrapod Limbs and Girdles: Fossil and Developmental Evidence. 2009.) Uppsala: Uppsala University, Evolutionary Organism; 2009. [Google Scholar]
- Boulet AM, Moon AM, Arenkiel BR, et al. The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol. 2004;273:361–372. doi: 10.1016/j.ydbio.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nowicki JL. A new view of patterning domains in the vertebrate mesoderm. Dev Cell. 2003;4:159–165. doi: 10.1016/s1534-5807(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nelson CE, Morgan BA, et al. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- Callier V, Clack JA, Ahlberg PE. Contrasting developmental trajectories in the earliest known tetrapod forelimbs. Science. 2009;324:364–367. doi: 10.1126/science.1167542. [DOI] [PubMed] [Google Scholar]
- Camp CL. Classification of the lizards. Bull Am Mus Nat Hist. 1923;48:298–481. [Google Scholar]
- Chan YF, Marks ME, Jones FC, et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327:302–305. doi: 10.1126/science.1182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe A, Phun HQ, Tieu DD, et al. Expression patterns of Hox10 paralogous genes during lumbar spinal cord development. Gene Expr Patterns. 2006;6:730–737. doi: 10.1016/j.modgep.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Clack JA. The origin of tetrapods. In: Heatwole H, Carroll RL, editors. Amphibian Biology. New York: Surrey Beatty; 2000. pp. 973–1029. Vol. 4, Paleontology; Chapter 2. [Google Scholar]
- Clack JA, Ahlberg PE. A New Stem Tetrapod from the Early Carboniferous of Northern Ireland. In Recent Advances in the Origin and Early Radiation of Vertebrates. München: Verlag Dr. Friedrich Pfeil; 2004. [Google Scholar]
- Clement G, Ahlberg PE, Blieck A, et al. Palaeogeography: Devonian tetrapod from western Europe. Nature. 2004;427:412–413. doi: 10.1038/427412a. [DOI] [PubMed] [Google Scholar]
- Coates MI. Hox genes, fin folds and symmetry. Nature. 1993;364:195–196. [Google Scholar]
- Coates MI. The origin of vertebrate limbs. Dev Suppl. 1994;1994:169–180. [PubMed] [Google Scholar]
- Coates MI. The tetrapod Acanthostega gunnari Jarvik: postcranial skeleton, basal tetrapod relationships and patterns of skeletal evolution. Trans R Soc Edinb Earth Sci. 1996;87:363–421. [Google Scholar]
- Coates MI. The evolution of paired fins. Theory Biosci. 2003;122:266–287. [Google Scholar]
- Coates MI, Clack JA. Polydactyly in the earliest known tetrapod limbs. Nature. 1990;347:66–69. [Google Scholar]
- Coates M, Cohn M. Fins, limbs, and tails: outgrowths and axial patterning in vertebrate evolution. BioEssays. 1998;000:371–381. [Google Scholar]
- Coates MI, Ruta M. Nice snakes, shame about the legs. TREE. 2000;15:503–507. doi: 10.1016/s0169-5347(00)01999-6. [DOI] [PubMed] [Google Scholar]
- Coates MI, Jeffery JE, Ruta M. Fins to limbs: what the fossils say. Evol Dev. 2002;4:390–401. doi: 10.1046/j.1525-142x.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Tickle C. Developmental basis of limblessness and axial patterning in snakes. Nature. 1999;399:474–479. doi: 10.1038/20944. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisúa-Belmonte JC, Abud H, et al. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995;80:739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Patel K, Krumlauf R, et al. Hox9 genes and vertebrate limb specification. Nature. 1997;387:97–101. doi: 10.1038/387097a0. [DOI] [PubMed] [Google Scholar]
- Cole NJ, Tanaka M, Prescott A, et al. Expression of limb initiation genes and clues to the morphological diversification of threespine stickleback. Curr Biol. 2003;13:R951–R952. doi: 10.1016/j.cub.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Cole NJ, Hall TE, Don EK, et al. Development and evolution of the muscles of the pelvic fin. PLoS Biol. 2011;9:e1001168. doi: 10.1371/journal.pbio.1001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Minowada G, MacArthur CA, et al. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- Cubbage CC, Mabee PM. Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae) J Morphol. 1996;229:121–160. doi: 10.1002/(SICI)1097-4687(199608)229:2<121::AID-JMOR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dahn RD, Davis MC, Pappano WN, et al. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature. 2007;445:311–314. doi: 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- Davenport J. Wing-loading, stability and morphometric relationships in flying fish (Exocoetidae) from the north-eastern Atlantic. J Mar Biol Assoc UK. 1992;72:25–39. [Google Scholar]
- Davenport J. How and why do flying fish fly? Rev Fish Biol Fisheries. 1994;4:184–214. [Google Scholar]
- Davenport J. Allometric constraints on stability and maximum size in flying fishes: implications for their evolution. J Fish Biol. 2003;62:455–463. [Google Scholar]
- DeLaurier A, Schweitzer R, Logan M. Pitx1 determines the morphology of muscle, tendon, and bones of the hindlimb. Dev Biol. 2006;299:22–34. doi: 10.1016/j.ydbio.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Deschamps J, van den Akker E, Forlani S, et al. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol. 1999;43:635–650. [PubMed] [Google Scholar]
- Don EK, Hall TE, Currie PD, et al. Morphology of pelvic fin loss in a zebrafish strain (Danio rerio. J Morphol. 2011;272:583–589. doi: 10.1002/jmor.10938. [DOI] [PubMed] [Google Scholar]
- Drucker EG, Lauder GV. Function of pectoral fins in rainbow trout: behavioral repertoire and hydrodynamic forces. J Exp Biol. 2003;206:813–826. doi: 10.1242/jeb.00139. [DOI] [PubMed] [Google Scholar]
- Duboc V, Logan MPO. Pitx1 is necessary for normal initiation of hindlimb outgrowth through regulation of Tbx4 expression and shapes hindlimb morphologies via targeted growth control. Development. 2011;138:5301–5309. doi: 10.1242/dev.074153. DOI: http://dev.biologists.org/content/138/24/5301.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durland JL, Sferlazzo M, Logan M, et al. Visualizing the lateral somitic frontier in the Prx1Cre transgenic mouse. J Anat. 2008;212:590–602. doi: 10.1111/j.1469-7580.2008.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Essex R. Studies in reptilian degeneration. Proc Zool Soc Lond. 1927;59:879–945. [Google Scholar]
- Fernandez-Teran M, Ros MA. The apical ectodermal ridge: morphological aspects and signaling pathways. Int J Dev Biol. 2008;52:857–871. doi: 10.1387/ijdb.072416mf. [DOI] [PubMed] [Google Scholar]
- Fink WL. Phylogenetic interrelationships of the stomiid fishes (Teleostei: Stomiiformes) Miscellaneous Publications Museum of Zoology, The University of Michigan. 1985;171:1–127. [Google Scholar]
- Fischer S, Draper BW, Neumann CJ. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development. 2003;130:3515–3524. doi: 10.1242/dev.00537. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Primate Adaptation and Evolution. 2nd edn. San Diego, CA: Academic Press; 1999. p. 596. [Google Scholar]
- Freitas R, Zhang G, Cohn MJ. Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature. 2006;442:1033–1037. doi: 10.1038/nature04984. [DOI] [PubMed] [Google Scholar]
- Gans C. Tetrapod limblessness: evolution and functional corollaries. Am Zool. 1975;15:455–467. [Google Scholar]
- Garcia-Fernandez J. Hox, ParaHox, ProtoHox: facts and guesses. Heredity. 2004;94:145–152. doi: 10.1038/sj.hdy.6800621. [DOI] [PubMed] [Google Scholar]
- Gaunt SJ. Conservation in the Hox code during morphological evolution. Int J Dev Biol. 1994;38:549–552. [PubMed] [Google Scholar]
- Gegenbaur C. Elements of Comparative Anatomy. London: Macmillan & Co; 1878. [Google Scholar]
- Geraudie J. Scanning electron microscope study of the developing trout pelvic fin bud. Anat Rec. 1978;191:391–396. doi: 10.1002/ar.1091910311. [DOI] [PubMed] [Google Scholar]
- Gibert Y, Gajewski A, Meyer A, et al. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649–2659. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- Gibson-Brown J, Agulnuk S, Chapman D, et al. Evidence of a role for T-box genes in the evolution of limb morphogenesis and the specification of forelimb/hindlimb identity. Mech Dev. 1996;56:93–101. doi: 10.1016/0925-4773(96)00514-x. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the Structure and Development of Vertebrates. Chicago: University of Chicago Press; 1930. [Google Scholar]
- Gosline WA. The evolution of some structural systems with reference to the interrelationships of modern lower teleostean fish groups. Jpn J Ichthyol. 1980;27:1–28. [Google Scholar]
- Goujet DF. Placoderms and Basal Gnathostome Apomorphies. London: Systematics Association; 2001. [Google Scholar]
- Grande L, Bemis WE. Osteology and phylogenetic relationships of fossil and recent paddlefishes (Polyodontidae) with comments on the interrelationships of Acipenseriformes. J Vert Paleontol. 1991;11:1–121. (Supplement to Number 1) [Google Scholar]
- Grandel H, Brand M. Zebrafish limb development is triggered by a retinoic acid signal during gastrulation. Dev Dyn. 2011;240:1116–1126. doi: 10.1002/dvdy.22461. [DOI] [PubMed] [Google Scholar]
- Grandel H, Schulte-Merker S. The development of the paired fins in the Zebrafish (Danio rerio. Mech Dev. 1998;79:99–120. doi: 10.1016/s0925-4773(98)00176-2. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch G, Jr, et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Greene HW, Cundall D. Limbless tetrapods and snakes with legs. Science. 2000;287:1939–1941. doi: 10.1126/science.287.5460.1939. [DOI] [PubMed] [Google Scholar]
- Greenwood PH, Rosen DE, Weitzman SH, et al. Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bull Am Mus Nat Hist. 1966;131:339–456. [Google Scholar]
- Gruss P, Kessel M. Axial specification in higher vertebrates. Curr Opin Genet Dev. 1991;1:204–210. doi: 10.1016/s0959-437x(05)80071-1. [DOI] [PubMed] [Google Scholar]
- Haas G. Pachyrhachis problematicus Haas, snakelike reptile from the Lower Cenomanian: ventral view of skull. Bull Mus Natl Hist Nat Paris. 1980;2:87–104. [Google Scholar]
- Harris JE. The mechanical significance of the position and movements of the paired fins in the Teleostei. Pap Tortugas Lab Carnegie Inst Wash. 1937;16:173–189. [Google Scholar]
- Harris JE. The role of the fins in the equilibrium of the swimming fish. II. The role of the pelvic fins. J Exp Biol. 1938;16:32–47. [Google Scholar]
- Hasson P, DeLaurier A, Bennett M, et al. Tbx4 and Tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell. 2010;18:148–156. doi: 10.1016/j.devcel.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Bremiller R, Westerfield M, et al. Diversity of expression of engrailed-like antigens in zebrafish. Development. 1991;112:821–832. doi: 10.1242/dev.112.3.821. [DOI] [PubMed] [Google Scholar]
- Howell AB. Aquatic Mammals. Their Adaptation to Life in the Water. New York: Dover Publications; 1970. [Google Scholar]
- Inoue JG, Miya M, Tsukamoto K, et al. Basal actinopterygian relationships: a mitogenomic perspective on the phylogeny of the ancient fish. Mol Phylogenet Evol. 2003;26:110–120. doi: 10.1016/s1055-7903(02)00331-7. [DOI] [PubMed] [Google Scholar]
- Janvier P. Early Vertebrates Monographs on Geology and Geophysics. New York: Oxford University Press; 1996. [Google Scholar]
- Jarvik E. Basic Structure and Evolution of Vertebrates. London: Academic Press; 1980. [Google Scholar]
- Jarvik E. The Devonian tetrapod Ichthyostega. Fossils Strata. 1996;40:1–213. [Google Scholar]
- Johanson Z. Evolution of paired fins and the lateral somitic frontier. J Exp Zoolog Part B Mol Dev Evol. 2010;314B:347–352. doi: 10.1002/jez.b.21343. [DOI] [PubMed] [Google Scholar]
- Johanson Z, Joss J, Boisvert CA, et al. Fish fingers: digit homologues in sarcopterygian fish fins. J Exp Zoolog Part B Mol Dev Evol. 2007;308B:757–768. doi: 10.1002/jez.b.21197. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Capdevila J, Büscher D, et al. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Khan P, Linkhart B, Simon HG. Different regulation of T-box genes Tbx4 and Tbx5 during limb development and limb regeneration. Dev Biol. 2002;250:383–392. [PubMed] [Google Scholar]
- King HM, Shubin NH, Coates MI, et al. Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes. Proc Natl Acad Sci USA. 2011;108:21146–21151. doi: 10.1073/pnas.1118669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301:331–333. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lamonerie T, Tremblay JJ, LanctÃ′t C, et al. Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev. 1996;10:1284–1295. doi: 10.1101/gad.10.10.1284. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, Omelchenko N, Bailis A, et al. Hoxd10 induction and regionalization in the developing lumbosacral spinal cord. Development. 2001;128:2255–2268. doi: 10.1242/dev.128.12.2255. [DOI] [PubMed] [Google Scholar]
- Lanctot C, Moreau A, Chamberland M, et al. Hindlimb patterning and mandible development require the Ptx1 gene. Development. 1999;126:1805–1810. doi: 10.1242/dev.126.9.1805. [DOI] [PubMed] [Google Scholar]
- Lauder GV, Drucker EG. Morphology and experimental hydrodynamics of fish fin control surfaces. IEEE J Oceanic Eng. 2004;29:556–571. [Google Scholar]
- Lebedev OA, Coates MI. The postcranial skeleton of the Devonian tetrapod Tulerpeton curtum Lebedev. Zool J Linnean Soc. 1995;114:307–348. [Google Scholar]
- Lee MSY, Caldwell MW. Anatomy and relationships of Pachyrhachis problematicus, a primitive snake with hindlimbs. Phil Trans R Soc Lond, Ser B. 1998;352:1521–1552. [Google Scholar]
- Lewandoski M, Sun X, Martin G. Fgf8 signalling from the AER is essential for normal limb development. Nat Gen. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Liem KF, Summers AP. Muscular System. Gross Anatomy and Functional Morphology of Muscles. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- Logan M. Finger or toe: the molecular basis of limb identity. Development. 2003;130:6401–6410. doi: 10.1242/dev.00956. DOI: http://dev.biologists.org/content/130/26/6401.long. [DOI] [PubMed] [Google Scholar]
- Logan M, Tabin CJ. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science. 1999;283:1736–1739. doi: 10.1126/science.283.5408.1736. [DOI] [PubMed] [Google Scholar]
- Logan M, Simon HG, Tabin C. Differential regulation of T-box and homeobox transcription factors suggests roles in controlling chick limbtype identity. Development. 1998;125:2825–2835. doi: 10.1242/dev.125.15.2825. [DOI] [PubMed] [Google Scholar]
- Mabee PM. Developmental data and phylogenetic systematics: evolution of the vertebrate limb. Am Zool. 2000;40:789–800. [Google Scholar]
- Mahmood R, Bresnick J, Hornbruch A, et al. A role for FGF-8 in the initiation and maintenance of vertebrate limb bud outgrowth. Curr Biol. 1995;5:797–806. doi: 10.1016/s0960-9822(95)00157-6. [DOI] [PubMed] [Google Scholar]
- Marcil A, Dumontier E, Chamberland M, et al. Pitx1 and Pitx2 are required for development of hindlimb buds. Development. 2003;130(1):45–55. doi: 10.1242/dev.00192. [DOI] [PubMed] [Google Scholar]
- Marshall H, Morrison A, Studer M, et al. Retinoids and Hox genes. FASEB J. 1996;10:969–978. [PubMed] [Google Scholar]
- Martin P. Tissue patterning in the developing mouse limb. Int J Dev Biol. 1990;34:323–336. [PubMed] [Google Scholar]
- Matsuura K. Phylogeny of the superfamily Balistoidea (Pisces: Tetraodontiformes) Mem Fac Fish Hokkaido Univ. 1979;26:49–169. [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- Menke DB, Guenther C, Kingsley DM. Dual hindlimb control elements in the Tbx4 gene and region-specific control of bone size in vertebrate limbs. Development. 2008;135:2543–2553. doi: 10.1242/dev.017384. [DOI] [PubMed] [Google Scholar]
- Mercader N, Fischer S, Neumann CJ. Prdm1 acts downstream of a sequential RA, Wnt and Fgf signaling cascade during zebrafish forelimb induction. Development. 2006;133:2805–2815. doi: 10.1242/dev.02455. [DOI] [PubMed] [Google Scholar]
- Minguillon C, Del Buono J, Logan MP. Tbx5 and Tbx4 are not sufficient to determine limb-specific morphologies but have common roles in initiating limb outgrowth. Dev Cell. 2005;8:75–84. doi: 10.1016/j.devcel.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Minguillon C, Gibson-Brown JJ, Logan MP. Tbx4/5 gene duplication and the origin of vertebrate paired appendages. Proc Natl Acad Sci U S A. 2009;106:21726–21730. doi: 10.1073/pnas.0910153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mivart SG. On the fins of Elasmobranchii. Trans Zool Soc London. 1879;10:439–484. [Google Scholar]
- Moon A, Capecchi M. Fgf8 is required for outgrowth and patterning of the limbs. Nat Gen. 2000;26:455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tanaka M. Evolution of motor innervation to vertebrate fins and limbs. Dev Biol. 2011;355:164–172. doi: 10.1016/j.ydbio.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Murata Y, Tamura M, Aita Y, et al. Allometric growth of the trunk leads to the rostral shift of the pelvic fin in teleost fishes. Dev Biol. 2010;347:236–245. doi: 10.1016/j.ydbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Murillo-Ferrol NL. Causal study of the earliest differentiation of the morphological rudiments of the extremities. Experimental analysis on bird embryos. Acta Anat (Basel) 1965;62:80–103. [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Tbx4 is not required for hindlimb identity or post-bud hindlimb outgrowth. Development. 2007;134:93–103. doi: 10.1242/dev.02712. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Arora R, Kania A, et al. Identity and fate of Tbx4-expressing cells reveal developmental cell fate decisions in the allantois, limb, and external genitalia. Dev Dyn. 2011;240:2290–2300. doi: 10.1002/dvdy.22731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T, Sasaoka S, Udagawa K, et al. Wnt10a is involved in AER formation during chick limb development. Dev Dyn. 2005;233:282–287. doi: 10.1002/dvdy.20321. [DOI] [PubMed] [Google Scholar]
- Narita T, Nishimatsu S-i, Wada N, et al. A Wnt3a variant participates in chick apical ectodermal ridge formation: distinct biological activities of Wnt3a splice variants in chick limb development. Dev Growth Differ. 2007;49:493–501. doi: 10.1111/j.1440-169X.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- Nelson JS. Analysis of the multiple occurrence of pelvic fin absence in extant fishes. Matsya. 1993;15/16:21–38. [Google Scholar]
- Nelson JS. Fishes of the World. New York: John Wiley & Sons; 1994. [Google Scholar]
- Nelson C, Morgan B, Burke A, et al. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- Neyt C, Jagla K, Thisse C, et al. Evolutionary origins of vertebrate appendicular muscle. Nature. 2000;408:82–86. doi: 10.1038/35040549. [DOI] [PubMed] [Google Scholar]
- Nicolas N, Gallien CL, Chanoine C. Expression of myogenic regulatory factors during muscle development of Xenopus: Myogenin mRNA accumulation if limited strictly to secondary myogenesis. Dev Dyn. 1998;213:309–321. doi: 10.1002/(SICI)1097-0177(199811)213:3<309::AID-AJA7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Niedzwiedzki G, Szrek P, Narkiewicz K, et al. Tetrapod trackways from the early Middle Devonian period of Poland. Nature. 2010;463:43–48. doi: 10.1038/nature08623. [DOI] [PubMed] [Google Scholar]
- Nomura R, Kamei E, Hotta Y, et al. Fgf16 is essential for pectoral fin bud formation in zebrafish. Biochem Biophys Res Commun. 2006;347:340–346. doi: 10.1016/j.bbrc.2006.06.108. [DOI] [PubMed] [Google Scholar]
- Noro M, Yuguchi H, Sato T, et al. Role of paraxial mesoderm in limb/flank regionalization of the trunk lateral plate. Dev Dyn. 2011;240:1639–1649. doi: 10.1002/dvdy.22666. [DOI] [PubMed] [Google Scholar]
- Norton WHJ, Ledin J, Grandel H, et al. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development. 2005;132:4963–4973. doi: 10.1242/dev.02084. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Nakagawa T, Yamauchi M, et al. An additional limb can be induced from the flank of the chick embryo by FGF4. Biochem Biophys Res Commun. 1995;209:809–816. doi: 10.1006/bbrc.1995.1572. [DOI] [PubMed] [Google Scholar]
- Ouimette J-Fo, Jolin ML, L'Honoré A, et al. Divergent transcriptional activities determine limb identity. Nat Commun. 2008;1:35. doi: 10.1038/ncomms1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, et al. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- Peters DS. Mechanical constraints canalizing the evolutionary transformation of tetrapod limbs. Acta Biotheor. 1985;34:157–164. [Google Scholar]
- Pierce SE, Clack JA, Hutchinson JR. Three-dimensional limb joint mobility in the early tetrapod Ichthyostega. Nature. 2012 doi: 10.1038/nature11124. doi: 10.1038/nature11124. [DOI] [PubMed] [Google Scholar]
- Presch W. The evolution of limb reduction in the teiid lizard genus. Bachia Bull So Calif Acad Sci. 1975;74:113–121. [Google Scholar]
- Raff RA. Written in stone: fossils, genes and evo-devo. Nat Rev Genet. 2007;8:911–920. doi: 10.1038/nrg2225. [DOI] [PubMed] [Google Scholar]
- Rage J-C, Escuillié F. Un nouveau serpent bipède du Cénomanien (Crétacé). Implications phylétiques. CR Acad Sci Paris. 2000;330:513–520. [Google Scholar]
- Raynaud A. Developmental mechanisms involved in the embryonic reduction of limbs in reptiles. Int J Dev Biol. 1990;34:233–243. [PubMed] [Google Scholar]
- Raynaud A, Okuzumi H, Kouprach S. Morphologie externe des stades précoces du développement des ébauches des membres du Lézard vert ‘Lacerta viridis Laur’ et de l'Orvet ‘Anguis fragilis L’, etudié au moyen de la microscopie électronique à balayage. Bull Soc Zool France. 1974;99:149–153. [Google Scholar]
- Reifers F, Bohli H, Walsh E, et al. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. DOI: http://dev.biologists.org/content/125/13/2381.long. [DOI] [PubMed] [Google Scholar]
- Rewcastle SC. Stance and gait in tetrapods: an evolutionary scenario. Symp Zool Soc Lond. 1981;48:239–267. [Google Scholar]
- Richardson M, Carl T, Hanken J, et al. Limb development and evolution: a frog embryo with no apical ectodermal ridge (AER) J Anat. 1998;192(Pt 3):379–390. doi: 10.1046/j.1469-7580.1998.19230379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, et al. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rieppel O. A review of the origin of snakes. Evol Biol. 1988;22:37–130. [Google Scholar]
- Rodriguez-Esteban C, Tsukui T, Yonei S, et al. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999;398:814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Rosen DE. Teleostean interrelationships, morphological function and evolutionary inference. Integr Comp Biol. 1982;22:261–273. [Google Scholar]
- Ruvinsky I, Gibson-Brown JJ. Genetic and developmental bases of serial homology in vertebrate limb evolution. Development. 2000;127:5233–5244. doi: 10.1242/dev.127.24.5233. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Oates AC, Silver LM, et al. The evolution of paired appendages in vertebrates: T-box genes in the zebrafish. Dev Genes Evol. 2000;210:82–91. doi: 10.1007/s004270050014. [DOI] [PubMed] [Google Scholar]
- Sabo MC, Nath K, Elinson RP. Lbx1 expression and frog limb development. Dev Genes Evol. 2009;219:609–612. doi: 10.1007/s00427-009-0314-8. [DOI] [PubMed] [Google Scholar]
- Santini F, Tyler JC. A phylogeny of the families of fossil and extant tetraodontiform fishes (Acanthomorpha, Tetraodontiformes), Upper Cretaceous to Recent. Zool J Linnean Soc. 2003;139:565–617. [Google Scholar]
- Satoh A, Sakamaki K, Ide H, et al. Characteristics of initiation and early events for muscle development in the Xenopus limb bud. Dev Dyn. 2005;234:846–857. doi: 10.1002/dvdy.20573. [DOI] [PubMed] [Google Scholar]
- Saunders JW. The proximo-distal sequence of origin of parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108:363–404. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Saunders JW. In: The experimental analysis of chick limb bud development. In Vertebrate Limb and Somite Morphogenesis. Ede DA, Hinchliffe JR, Balls M, editors. Cambridge: Cambridge University Press; 1977. pp. 1–25. [Google Scholar]
- Saunders JJ, Gasseling M. Ectoderm-mesenchymal interaction in the origins of wing symmetry. In: Fleischmajer R, Billingham R, editors. Epithelial-Mesenchymal Interactions. Baltimore: Williams & Wilkins; 1968. pp. 289–314. [Google Scholar]
- Schrank AJ, Webb PW, Mayberry S. How do body and paired-fin positions affect the ability of three teleost fishes to maneuver around bends? Can J Zool. 1999;77:203–210. [Google Scholar]
- Sedmera D, Misek I, Klima M. On the development of cetacean extremities. I. Hind limb rudimentation in the spotted dolphin (Stenella attenuata. Eur J Morphol. 1997a;35:25–30. doi: 10.1076/ejom.35.1.25.13058. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Misek I, Klima M. On the development of cetacean extremities. II. Morphogenesis and histogenesis of the flippers in the spotted dolphin (Stenella attenuata. Eur J Morphol. 1997b;35:117–123. doi: 10.1076/ejom.35.2.117.13067. [DOI] [PubMed] [Google Scholar]
- Shapiro MD, Bell MA, Kingsley DM. Parallel genetic origins of pelvic reduction in vertebrates. Proceedings of the National Academy of Sciences. 2006;103:13753–13758. doi: 10.1073/pnas.0604706103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MD, Marks ME, Peichel CL, et al. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature. 2004;428:717–723. doi: 10.1038/nature02415. [DOI] [PubMed] [Google Scholar]
- Shubin N. The evolution of paired fins and the origin of tetrapod limbs phylogenetic and transformational approaches. Evol Biol. 1995;28:39–86. [Google Scholar]
- Sordino P, van der Hoeven F, Duboule D. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature. 1995;375:678–681. doi: 10.1038/375678a0. [DOI] [PubMed] [Google Scholar]
- Standen EM. Pelvic fin locomotor function in fishes: three-dimensional kinematics in rainbow trout (Oncorhynchus mykiss. J Exp Biol. 2008;211:2931–2942. doi: 10.1242/jeb.018572. [DOI] [PubMed] [Google Scholar]
- Stephens TD, McNulty TR. Evidence for a metameric pattern in the development of the chick humerus. J Embryol Exp Morphol. 1981;61:191–205. [PubMed] [Google Scholar]
- Stiassny MLJ, Moore JA. A review of the pelvic girdle of acanthomorph fishes, with comments on hypotheses of acanthomorph intrarelationships. Zool J Linnean Soc. 1992;104:209–242. [Google Scholar]
- Stopper GF, Wagner GP. Of chicken wings and frog legs: A smorgasbord of evolutionary variation in mechanisms of tetrapod limb development. Dev Biol. 2005;288:21–39. doi: 10.1016/j.ydbio.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Swartz B. A marine stem-tetrapod from the Devonian of Western North America. PLoS One. 2012;7(3):e33683. doi: 10.1371/journal.pone.0033683. doi: 10.1371/journal.pone.0033683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney RM, Watterson RL. Rib development in chick embryos analyzed by means of tantalum foil blocks. Am J Anat. 1969;126:127–149. doi: 10.1002/aja.1001260202. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Rodriguez-Esteban Cn, Ryan AK, et al. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 1999;13:484–494. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C, Laufer E. Hox genes and serial homology. Nature. 1993;361:692–693. [Google Scholar]
- Takabatake Y, Takabatake T, Takeshima K. Conserved and divergent expression of T-box genes Tbx2–Tbx5 in Xenopus. Mech Dev. 2000;91:433–437. doi: 10.1016/s0925-4773(99)00329-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Koshiba-Takeuchi K, Matsumoto K, et al. Tbx5 and Tbx4 genes determine the wing/leg identity of limb buds. Nature. 1999;398:810–814. doi: 10.1038/19762. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Koshiba-Takeuchi K, Suzuki T, et al. Tbx5 and Tbx4 trigger limb initiation through activation of the Wnt/Fgf signaling cascade. Development. 2003;130:2729–2739. doi: 10.1242/dev.00474. [DOI] [PubMed] [Google Scholar]
- Tamura K, Yonei-Tamura S, Belmonte JCI. Differential expression of Tbx4 and Tbx5 in zebrafish fin buds. Mech Dev. 1999;87:181–184. doi: 10.1016/s0925-4773(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Munsterberg A, Anderson WG, et al. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature. 2002;416:527–531. doi: 10.1038/416527a. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hale LA, Amores A, et al. Developmental genetic basis for the evolution of pelvic fin loss in the pufferfish Takifugu rubripes. Dev Biol. 2005;281:227–239. doi: 10.1016/j.ydbio.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Tchernov E, Rieppel O, Zaher H, et al. A fossil snake with limbs. Science. 2000;287:2010–2012. doi: 10.1126/science.287.5460.2010. [DOI] [PubMed] [Google Scholar]
- Thacher JK. Median and paired fins, a contribution to the history of vertebrate limbs. Trans Conn Acad. 1877;3:281–310. [Google Scholar]
- Thewissen JGM, Cohn MJ, Stevens LS, et al. Developmental basis for hind-limb loss in dolphins and origin of the cetacean bodyplan. Proc Natl Acad Sci U S A. 2006;103:8414–8418. doi: 10.1073/pnas.0602920103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood P, Ferretti P. Hox genes, fin folds and symmetry. Nature. 1993;364:196. (15 July 1993); doi: 10.1038/364196a0. [Google Scholar]
- Tschumi PA. The growth of the hindlimb bud of Xenopus laevis and its dependence upon the epidermis. J Anat. 1957;91:149–173. [PMC free article] [PubMed] [Google Scholar]
- Tyler JC. Osteology, phylogeny, and higher classification of the fishes of the order Plectognathi (Tetraodontiformes) NOAA Tech Rep NMFS Circular. 1980;434:1–422. [Google Scholar]
- Vasyutina E, Birchmeier C. The development of migrating muscle precursor cells. Anat Embryol. 2006;211:37–41. doi: 10.1007/s00429-006-0118-9. [DOI] [PubMed] [Google Scholar]
- Vogel A, Rodriguez C, Izpisua-Belmonte J. Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development. 1996;122:1737–1750. doi: 10.1242/dev.122.6.1737. [DOI] [PubMed] [Google Scholar]
- Vorobyeva EL. The Fin-limb Transformation: Paleontological and Embryological Evidence. New York: Plenum Press; 1991. [Google Scholar]
- Vorobyeva EI, Hinchliffe RJ. The fin-limb transition and sarcopterygian relationships. Geobios. 1995;28(Suppl. 2):289–292. [Google Scholar]
- Wagner GP, Chiu C-H. The tetrapod limb: a hypothesis on its origin. J Exp Zool. 2001;291:226–240. doi: 10.1002/jez.1100. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Larsson HCE. Fins and Limbs in the Study of Evolutionary Novelties. Chicago: The University of Chicago Press; 2007. [Google Scholar]
- Walker WF, Homberger DG. Vertebrate Dissection. 8th edn. Fort Worth: Saunders Publishing Company; 1992. [Google Scholar]
- Webb PW. Locomotor patterns in the evolution of actinopterygian fishes. Am Zool. 1982;22:329–342. [Google Scholar]
- Webb PW. The Biology of Fish Swimming. New York: Cambridge University Press; 1996. [Google Scholar]
- Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Philadelphia. 1974;125:225–317. [Google Scholar]
- Yamanoue Y, Setiamarga DHE, Matsuura K. Pelvic fins in teleosts: structure, function and evolution. J Fish Biol. 2010;77:1173–1208. doi: 10.1111/j.1095-8649.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Sasaki H, Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991;353:443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- Yonei-Tamura S, Endo T, Yajima H, et al. FGF7 and FGF10 directly induce the apical ectodermal ridge in chick embryos. Dev Biol. 1999;211:133–143. doi: 10.1006/dbio.1999.9290. [DOI] [PubMed] [Google Scholar]
- Yonei-Tamura S, Abe G, Tanaka Y, et al. Competent stripes for diverse positions of limbs/fins in gnathostome embryos. Evol Dev. 2008;10:737–745. doi: 10.1111/j.1525-142X.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- Young GC, Barwick RE, Campbell KSW. Pelvic girdles of lungfishes (Dipnoi) In: LeMaitre RW, editor. Pathways in Genology: Essays in Honour of Edwin Sherbon Hills. Melbourne: Blackwell Scientific; 1989. pp. 59–75. [Google Scholar]
- Zangerl R. Handbook of Paleoichthyology, Chondrichthyans. Stuttgart: Gustav Fischer Verlag; 1981. [Google Scholar]
- Zhao X, Sirbu IO, Mic FA, et al. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr Biol. 2009;19:1050–1057. doi: 10.1016/j.cub.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Yu X, Choo B, et al. An antiarch placoderm shows that pelvic girdles arose at the root of jawed vertebrates. Biol Lett. 2012;8:453–456. doi: 10.1098/rsbl.2011.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]


