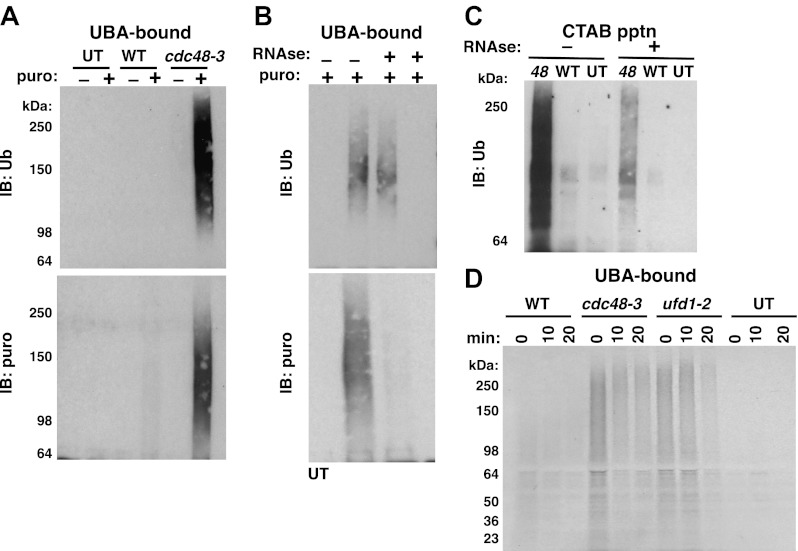
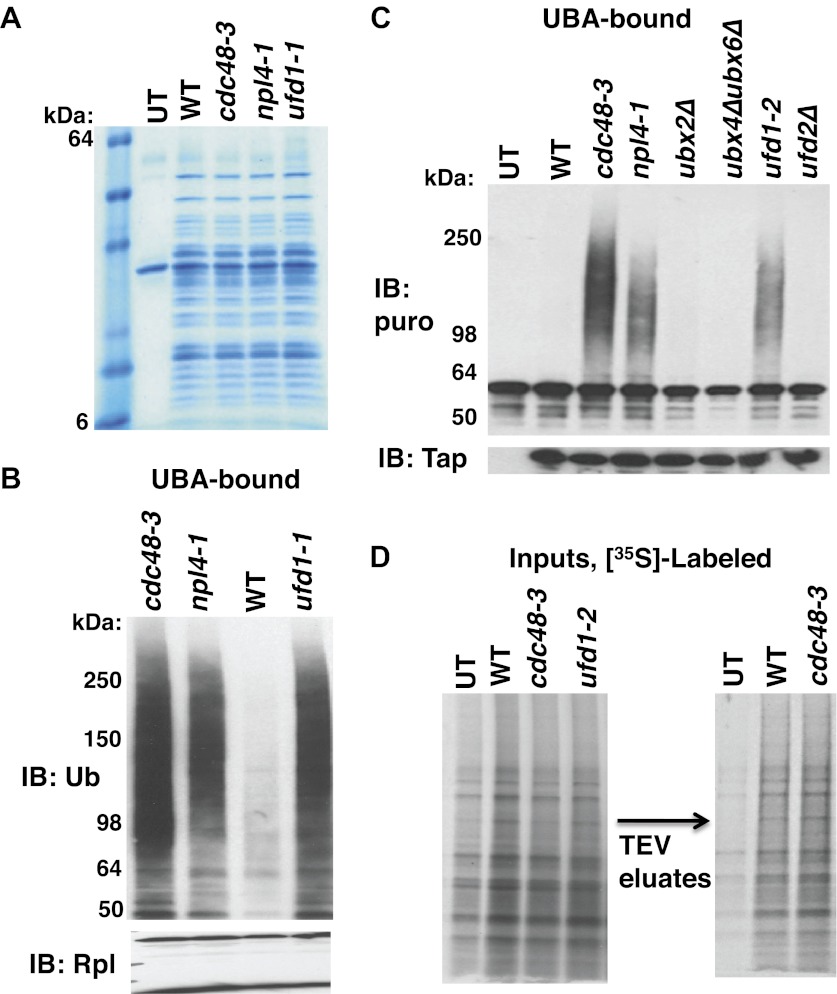
Figure 2. Ubiquitinated nascent peptides linked to tRNA accumulate on ribosomes in cdc48-3 and ufd1-2 mutants.
(A) Puromycin-dependent binding to UBA resin of Ub conjugates from cdc48-3 ribosomes. Ribosomes were affinity-purified from untagged or wildtype and mutant RPL18BTAP cells grown at 37°C for 90 min and eluted with TEV protease in the presence or absence of puromycin (puro), followed by incubation with UBA resin. Bound fractions were resolved by SDS-PAGE and immunoblotted with antibodies to Ub (top panel) or puromycin (lower panel). (B) Transfer of Ub conjugates to puromycin was RNAse A-sensitive. Ribosomes were affinity-purified from cdc48-3 (UT) or cdc48-3RPL18BTAP cells as described above in the absence (lanes 1, 2 and 3) or presence of 200 μg/ml RNAse A. Following elution with TEV protease, samples from the tagged cells (lane 3) were treated with 200 μg/ml RNAse A at 30°C for 10 min before incubation of all samples with puromycin and binding to UBA resin. The bound fractions were evaluated as in (A). (C) CTAB-precipitable Ub conjugates accumulate on cdc48-3 ribosomes. Ribosomes affinity-purified from the same strains used in panel (A) were treated with RNAse A (or not) and subjected to precipitation (pptn) with CTAB. Precipitates were resolved by SDS-PAGE and immunoblotted with anti-Ub. 48 Refers to cdc48-3. (D) Ubiquitinated newly-synthesized proteins accumulate on ribosomes isolated from cdc48-3 and ufd1-2 mutants. Cells were pulse-labeled for 90 s with 35S methionine and chased with cold methionine and cycloheximide for the indicated times. Ribosomes were affinity-purified, eluted with TEV protease in the presence of puromycin, and loaded onto UBA resin. The bound fraction was evaluated by SDS-PAGE followed by autoradiography. Densitometry indicated that <5% of the high MW material was released from ufd1-2 ribosomes between the 0- and 10-min time points.