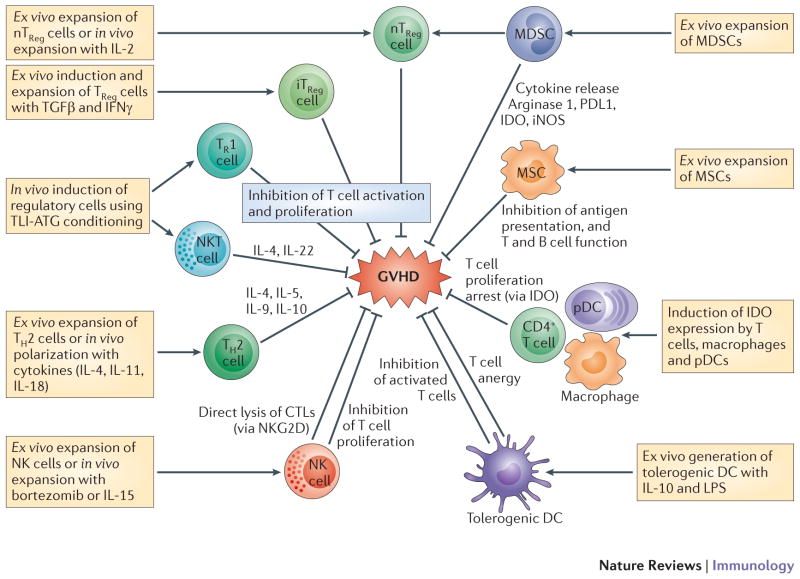
Figure 5. Potential targets for cellular immunotherapies in GVHD.
Tregs are either formed naturally by thymic differentiation (nTRegs) or are induced in the periphery from naive T-cells. Induced Tregs (iTRegs) can be divided into IL4, IL-10 and TGFβ-producing Th3 cells (CD4+CD25+FOXP3+) and CD25- but CD4+FOXP3+ iTregs that also produce IL-10 and TGFβ. FOXp3- Tr1 cells produce IL-10 and have shown potent suppressive effects on GVHD in the context of total lymphoid irradiation and anti-thymocyte globulin (TLI-ATG) conditioning regimen which also induces the generation of IL4-producing NKT cells. Ex vivo expanded Th2 polarized cell are already in clinical trials for the treatment of aGVHD. NK cells trials are also underway using NK cell infusion or activations of NK cells in vivo to delete alloreactive T cells. Substantial data on suppressive effects of IDO+ T cells, macrophages and DCs, make them prime candidates for future clinical trials. Third party infusion of mesenchymal stem cells (MSCs) for the treatment of GVHD, has created mixed results. Transfer of donor-derived Tregs expanded ex vivo has been more promising. Infusion of ex vivo expanded Myeloid derived stem cells (MDSCs) in pre-clinical models using G-& GM-CSF +/− IL-13 has shown to be feasible with anti-GVHD effect. Injection of pegylated-arginase may have the same benefit and is more practical therapeutically.