SUMMARY
Store-operated Ca2+ entry (SOCE) and Ca2+ release-activated Ca2+ currents (Icrac) are strongly suppressed during mitosis, the only known physiological situation in which Ca2+ store depletion is uncoupled from the activation of Ca2+ influx. We found that the ER Ca2+ sensor, STIM1, failed to rearrange into near-plasma membrane puncta in mitotic cells, a critical step in the SOCE activation pathway. We also found that STIM1 from mitotic cells is recognized by the phosphospecific MPM-2 antibody, suggesting that STIM1 is phosphorylated during mitosis. Removal of 10 MPM-2-recognition sites by truncation at amino acid 482 abolished MPM-2 recognition of mitotic STIM1, and significantly rescued STIM1 rearrangement and SOCE responses in mitosis. We identified S486 and S668 as mitosis-specific phosphorylation sites, and STIM1 containing mutations of these sites to alanine also significantly rescued mitotic SOCE. Therefore, phosphorylation of STIM1 at S486 and S668, and possibly other sites, underlies suppression of SOCE during mitosis.
INTRODUCTION
Cells utilize Ca2+ as an important second messenger in the regulation of many processes including exocytosis, immune cell activation, and cell division.1 The cell utilizes two main sources of Ca2+: the extracellular medium and intracellular Ca2+ storage organelles, most notably the endoplasmic reticulum (ER). Ultimately, the ER must utilize extracellular Ca2+ to maintain stored Ca2+ through store-operated Ca2+ entry (SOCE).2, 3 SOCE is not only important for maintaining the ability of the ER to support subsequent Ca2+ release events, but is also an important signaling mechanism, and it is becoming increasingly clear that certain processes are activated exclusively by Ca2+ that enters the cell via the SOCE pathway.4, 5
The most ubiquitous and well-defined SOCE involves the Ca2+ release-activated Ca2+ current (Icrac).3, 6 Two families of proteins are required for Icrac activation: STIM (STIM1 and STIM2)7, 8 Ca2+ sensor proteins, and Orai (Orai1, 2 and 3)9–11 Ca2+ channel subunits. Upon Ca2+ store depletion, STIM1 relocalizes into punctate structures within the ER near the plasma membrane.8, 12, 13 This dramatic rearrangement of STIM1 brings it into close apposition to the plasma membrane, where it interacts with and activates Orai SOCE channels.14, 15 By definition, SOCE is regulated primarily by ER Ca2+ store levels. However, whether this process is modified by other signaling processes is not clear.3 The only known physiological situation in which SOCE appears to be strongly negatively regulated is during mitosis.16–19 However, the mechanism by which this suppression occurs is unknown. Here we demonstrate that phosphorylation of STIM1 specifically during mitosis supresses SOCE during cell division. These findings represent the first demonstration of a specific role of STIM1 phosphorylation, with significant implications for the process of SOCE activation. They also contribute to our understanding of the role that Ca2+ signaling plays during cell division, a topic that has long been a black box of cell biology.20–22
RESULTS AND DISCUSSION
SOCE is suppressed in mitotic HeLa and HEK293 cells independently of changes in Orai1 or STIM1 expression
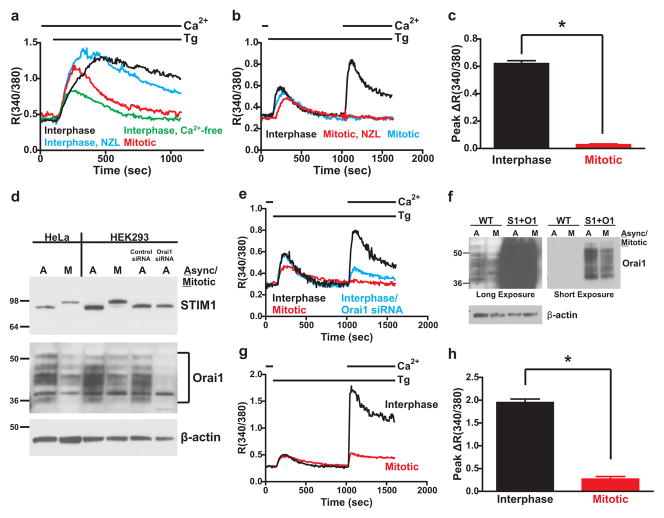
In interphase HeLa cells, treatment with the sarco/endoplasmic reticulum ATPase inhibitor thapsigargin resulted in a sustained rise in cytoplasmic Ca2+ indicative of SOCE (Figure 1a). In the absence of extracellular Ca2+, sustained Ca2+ elevation was lost, confirming that this sustained phase is due to Ca2+ influx. For synchronization in mitosis HeLa cells were treated with 1.67 μM nocodazole for 16 hours,23 after which approximately 50% of the cells appeared mitotic based on spherical morphology. As previously reported,16, 18 mitotic HeLa cells showed no sustained Ca2+ elevation, whereas the flat interphase cells on the same coverslip exhibited normal sustained Ca2+ elevation. For HEK293 cells, nocodazole-arrested mitotic cells were treated with thapsigargin in the absence of extracellular Ca2+ to deplete Ca2+ stores, followed by restoration of extracellular Ca2+ to reveal SOCE. Restoration of extracellular Ca2+ resulted in a large increase in cytoplasmic Ca2+ in interphase cells, indicative of SOCE; this response was again absent in the nocodazole-treated mitotic cells or in round mitotic cells identified from non-nocodazole-treated asynchronous populations (Figure 1b,c).
Figure 1. SOCE is suppressed in mitotic HeLa and HEK293 cells independently of changes to STIM1 and Orai1 expression.
a) SOCE was measured in HeLa cells by treating cells with thapsigargin (Tg; 2 μM) in the continuous presence of 1.8 mM extracellular Ca2+ as indicated (except green trace). For controls, interphase cells were selected from an asynchronous population based on their flat, well-spread morphology (black trace). For comparison, cells treated with thapsigargin in the presence of nominally Ca2+-free external solution failed to exhibit a sustained SOCE response (green trace). Nocodazole (NZL)-arrested mitotic cells were selected based on their spherical morphology (red trace), and flat interphase cells were also selected from the same population of nocodazole-treated cells (blue trace). b) SOCE was measured in HEK293 cells by treating cells with thapsigargin in nominally Ca2+-free external solution, followed by restoration of 1.8 mM external Ca2+ as indicated. Interphase cells (black trace) were selected from asynchronous populations, whereas mitotic cells were arrested by nocodazole treatment (red trace) or were identified from asynchronous populations based on their round morphology (blue trace). Each trace represents the averaged response from 20–30 cells from a single experiment. c) The peak increase in fluorescence ratio above baseline following Ca2+ add-back was calculated and averaged for each cell from experiments as described in panel b. Interphase: n = 90 cells, 3 coverslips; Mitotic (nocodazole-arrested): n = 88 cells, 3 coverslips. ‘*’ indicates statistically significant difference (t-test; p < 0.0001). Error bars represent S.E.M. d) Total protein lysates from asynchronous or nocodazole-arrested mitotic HeLa (lanes 1 and 2) and HEK293 (lanes 3 and 4) cells were analyzed by Western blot with anti-STIM1 (upper panel), anti-Orai1 (middle panel) and anti-β-actin (lower panel) antibodies. The same blot was stripped and reprobed between each antibody. Also analyzed were lysates from asynchronous HEK293 cells treated with control siRNA or Orai1-specific siRNA (Lanes 5 and 6). Full scans of these blots are shown in Supplemental Figure 6a. Note the upward shift in apparent molecular weight of STIM1 from mitotic compared to asynchronous samples. Orai1 appeared as a series of differentially glycosylated bands between 36 and 50 kDa. The multiple banding pattern of Orai1 is likely due to the presence of multiple patterns of N-glycosylation, because treatment of cell lysates with N-glycosidase F causes a loss of this banding pattern and the appearance of distinct Orai1 bands (Supplemental Figure 1). e) SOCE responses in HEK293 cells upon 1.8 mM Ca2+ add-back following store depletion with thapsigargin (Tg; 2 μM). Black trace: interphase cells selected from an asynchronous population; blue trace: interphase cells selected from an asynchronous, Orai1 siRNA-treated population; red trace: nocodazole-arrested mitotic cells. Each trace represents the averaged response of 20–30 cells from a single experiment. f) Western blot analysis of Orai1 protein expression in total lysates from wildtype (WT; lanes 1 and 2) or eYFP-STIM1 and Orai1 overexpressing (S1+O1; lanes 3 and 4) asynchronous and mitotic HEK293 cells. Because of the substantial difference in protein expression levels in wildtype and overexpressing samples, a long and short exposure of the same blot are shown. β-actin is shown as a loading control. A full scan of the Orai1 blot is shown in Supplemental Figure 6b. g) SOCE responses in interphase (black trace) or mitotic (red trace) eYFP-STIM1 and Orai1 overexpressing HEK293 cells as described in panel e, except Ca2+ add-back was 1.0 mM. h) The peak increase in fluorescence ratio above baseline following Ca2+ add-back was calculated and averaged for each cell from experiments as described in panel c. Interphase: n = 86 cells, 3 coverslips; Mitotic: n = 75 cells, 3 coverslips. ‘*’ indicates statistically significant difference (t-test; p < 0.0001). Error bars represent S.E.M.
STIM1 expression was not altered in mitotic HeLa or HEK293 cell lysates (Figure 1d). However, there was a striking increase in the apparent molecular weight of mitotic STIM1, likely due to STIM1 phosphorylation. In contrast, expression of Orai1 was reduced in mitotic cells by approximately 25–50%. However, the loss of SOCE in mitosis cannot solely be due to a decrease in Orai1. First, Orai1 siRNA in asynchronous cells decreased protein levels to a greater extent than the decrease in mitotic cells (Figure 1d), but inhibited Ca2+ entry to a lesser extent (Figure 1e). Second, transfection of STIM1 and Orai1 greatly increased Orai1 expression in mitotic cells, produced large increases in SOCE in interphase cells, but did not rescue SOCE in mitotic cells (Figure 1f–h, Supplemental Figure 2a).
STIM1 rearrangement is suppressed in mitosis
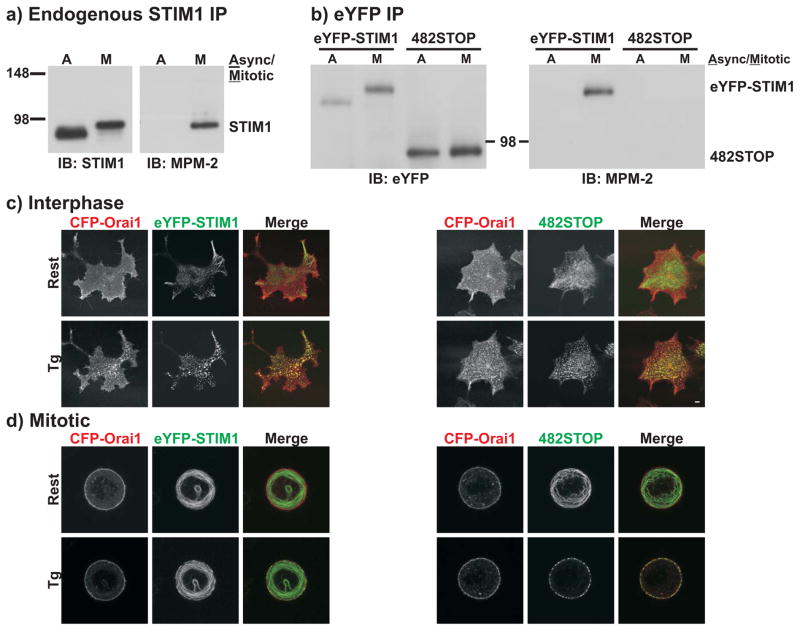
STIM1 localization and function depend on microtubules.24, 25 Thus, remodeling of the microtubule cytoskeleton into the mitotic spindle during mitosis may contribute to SOCE suppression26. In contrast to interphase HeLa cells in which extensive colocalization of eYFP-STIM1 with α-tubulin was observed, eYFP-STIM1 localization was completely dissociated from that of α-tubulin in mitotic HeLa cells (Figure 2a). Additionally, there was a complete lack of eYFP-STIM1 rearrangement into puncta in response to Ca2+ store depletion in mitotic HEK293 (Figure 2b,c) and HeLa (Supplemental Figure 3) cells. This lack of STIM1 rearrangement can readily account for the failure of SOCE during mitosis. However, because dissociation of STIM1 from microtubules does not prevent STIM1 puncta formation 25, mechanisms other than microtubule dissociation must contribute to SOCE inhibition.
Figure 2. STIM1 localization patterns are altered in mitosis.
a) Confocal imaging was carried out with eYFP-STIM1 expressing HeLa cells that were fixed and immunostained for α-tubulin and stained with DAPI. The upper panel shows a single image plane of an interphase cell. In the lower panel, a series of 128 planar images of a mitotic cell was deconvolved and a resultant image plane from near the center of the cell is shown. The mitotic cell was selected from an asynchronous population and was not nocodazole-arrested. In the merged images, eYFP-STIM1 is shown in red, α-tubulin is shown in green, and co-localization appears yellow. b and c) Confocal images of eYFP-STIM1 in live interphase or nocodazole-arrested mitotic HEK293 cells prior to (Stores Full) and following Ca2+ store depletion with thapsigargin (2 μM). Both equatorial (upper panels) and cortical (lower panels) slices of the same cells are shown. Scalebars are 5 μm.
STIM1 is phosphorylated during mitosis
The increase in apparent size of STIM1 during mitosis suggests phosphorylation.27, 28 Thus, we immunoprecipitated STIM1 from asynchronous or nocodazole-arrested mitotic HeLa cells and probed with MPM-2 antibody, which recognizes phosphorylated serine or threonine followed immediately by proline.29–31 Endogenous STIM1 or expressed eYFP-STIM1 from mitotic HeLa cells was recognized by MPM-2; proteins from asynchronous cells were not. The blots were reprobed for STIM1 or eYFP-STIM1, showing the band shift of mitotic STIM1 (Figure 3a,b). Identical results were also obtained with eYFP-STIM1 from mitotic HeLa cells isolated from non-nocodazole treated, asynchronous populations (Supplemental Figure 4a). Thus, STIM1 is phosphorylated during mitosis at one or more MPM-2 sites. eYFP-STIM2 was also recognized by MPM-2 in mitotic but not asynchronous immunoprecipitates (Supplemental Figure 4c).
Figure 3. STIM1 is phosphorylated during mitosis.
a) Endogenous STIM1 was immunoprecipitated from asynchronous or mitotic HeLa cell lysates and analyzed by Western blot with the phosphospecific MPM-2 antibody (right panel). The blot was then stripped and re-probed with an anti-STIM1 antibody (left panel) to reveal total STIM1 amounts. Full scans of these blots are shown in Supplemental Figure 6c. b) eYFP-STIM1 or 482STOP were immunoprecipitated from asynchronous or nocodazole-arrested mitotic HeLa cell lysates with an anti-eYFP antibody and analyzed by Western blot with the MPM-2 antibody (right panel). The blot was then stripped and re-probed with the anti-eYFP antibody to reveal total protein amounts (left panel). Full scans of these blots are shown in Supplemental Figure 6d. Although most STIM1 phosphorylation experiments were carried out with HeLa cells (see Methods), we also observed recognition of eYFP-STIM1 by MPM-2 in mitotic HEK293 cells (Supplemental Fig. 4b). c and d) Confocal images of interphase and nocodazole-arrested mitotic HEK293 cells co-expressing eYFP-STIM1 and CFP-Orai1 (left panels) or 482STOP and CFP-Orai1 (right panels) prior to (Rest) and following Ca2+ store depletion with thapsigargin (Tg; 2 μM). In merged images, CFP-Orai1 is red, eYFP-STIM1 or 482STOP are green, and co-localization is yellow. Scalebars are 5 μm. Images are representative of greater than 5 cells for each condition.
Truncation of STIM1 phosphorylation sites rescues SOCE in mitosis
Human STIM1 contains ten serines or threonines followed by proline, the minimal recognition sequence for MPM-2, all within the cytoplasmic, C-terminus (Supplemental Figure 5). Given the large number of possible phosphorylation sites and the likelihood that multiple sites contribute to SOCE suppression, we initially employed a truncation approach to examine the role of this C-terminal region. Truncation of eYFP-STIM1 at amino acid 482 (482STOP) resulted in a protein that was not recognized by MPM-2 (Figure 3b). Confocal imaging revealed that upon Ca2+ store depletion in interphase cells, 482STOP formed near-plasma membrane puncta that co-localized with CFP-Orai1, indistinguishable from rearrangement of full-length eYFP-STIM1 (Figure 3c). In mitotic cells, the localization of full-length eYFP-STIM1 was unaltered following Ca2+ store depletion, while there was substantial and near-complete rearrangement of 482STOP to the plasma membrane upon Ca2+ store depletion (Figure 3d). This rearranged 482STOP co-localized with CFP-Orai1. Therefore, truncation of eYFP-STIM1 and subsequent loss of phosphorylation sites resulted in rearrangement into puncta in mitotic cells, consistent with the hypothesis that STIM1 phosphorylation underlies prevention of STIM1 rearrangement.
In interphase cells, 482STOP+Orai1 resulted in SOCE responses that were indistinguishable from those with full-length eYFP-STIM1+Orai1 (Figure 4a,b), indicating that 482STOP supports SOCE activity despite the large truncation. Importantly, mitotic HEK293 cells with 482STOP+Orai1 exhibited SOCE that was substantially greater than that from mitotic eYFP-STIM1+Orai1 cells (Figure 4a,b, Supplemental Figure 2b).
Figure 4. 482STOP rescues SOCE and Icrac responses in mitotic cells.
a) SOCE responses to restoration of 1.0 mM external Ca2+ following Ca2+ store depletion with thapsigargin (Tg; 2 μM) were measured in interphase (black trace) and nocodazole-arrested mitotic (gray trace) HEK293 cells co-expressing eYFP-STIM1 and Orai1 (S1+O1), and interphase (dark blue) and mitotic (light blue) cells co-expressing 482STOP and Orai1 (482STOP+O1). Each trace represents the average response of 20–30 cells from a single experiment. b) The peak increase in fluorescence ratio above baseline following Ca2+ add-back was calculated and averaged for each cell from experiments as described in panel a. Interphase eYFP-STIM1+Orai1: n = 79 cells, 3 coverslips; interphase 482STOP+Orai1: n = 66 cells, 3 coverslips; mitotic eYFP-STIM1+Orai1: n = 53 cells, 3 coverslips; mitotic 482STOP+Orai1: n = 47 cells, 3 coverslips. ‘*’ indicates statistically significant difference (one-way ANOVA followed by Tukey-Kramer; p < 0.05). Error bars represent S.E.M. Whole-cell Icrac currents were measured in interphase (c) and nocodazole-arrested mitotic (d) HEK293 cells co-expressing eYFP-STIM1 and Orai1 (S1+O1; black traces) and 482STOP and Orai1 (482STOP+O1; blue traces). The whole-cell condition was achieved in the presence of 2.0 mM external Ca2+, followed by switch to a divalent-free (DVF) external solution at 60 seconds. External Ca2+ was restored at 120 seconds, and 5 μM Gd3+ was added at 180 seconds to demonstrate full inhibition of Icrac currents. Current densities measured at −100 and +100 mV are plotted, and each trace represents the response of a single cell. Current-voltage relationships for the Ca2+ currents just prior to the switch to DVF and for the peak DVF currents are shown on the right. Peak leak-subtracted Ca2+ (e) and Na+ (f) currents measured at −100 mV for each cell were averaged and plotted. Interphase eYFP-STIM1+Orai1: n = 9 cells; interphase 482STOP+Orai1: n = 13 cells; mitotic eYFP-STIM1+Orai1: n = 11 cells; mitotic 482STOP+Orai1: n = 14 cells. ’*’ indicates statistically significant difference (t-test, p < 0.0001). Error bars represent S.E.M.
HEK293 cells co-expressing eYFP-STIM1+Orai1 exhibited inward Ca2+ currents at −100 mV of −10 to −20 pA/pF (Figure 4c,e). These currents were inwardly-rectifying with reversal potentials greater than +50 mV, and switch to divalent-free (DVF) external solution resulted in an inwardly-rectifying Na+ conductance that was approximately five times the Ca2+ conductance (Figure 4c,f), all well-documented properties of Icrac. Ca2+ and Na+ conductances were completely absent in 9 of 11 mitotic eYFP-STIM1+Orai1 cells (Figure 4c,e,f). Ca2+ current densities in interphase 482STOP+Orai1 cells were smaller than those in eYFP-STIM1 and Orai1 cells (Figure 4c,e). Analysis of current-voltage relationships from interphase 482STOP+Orai1 cells revealed that Ca2+ conductances were reduced at negative potentials, but Na+ conductances were unaffected, suggesting an unknown effect on a Ca2+-dependent property of Orai1 channels due to the STIM1 truncation. Nonetheless, 7 of 14 mitotic 482STOP+Orai1 cells exhibited Ca2+ currents of 1 pA/pF or greater (Figure 4d,e), and 12 of 14 cells exhibited clear inwardly-rectifying Na+ currents (Figure 4d,f). The average Ca2+ and Na+ current densities in mitotic 482STOP+Orai1 cells were not significantly different from those in interphase cells (Figure 4e,f)
Individual STIM1 phosphorylation sites
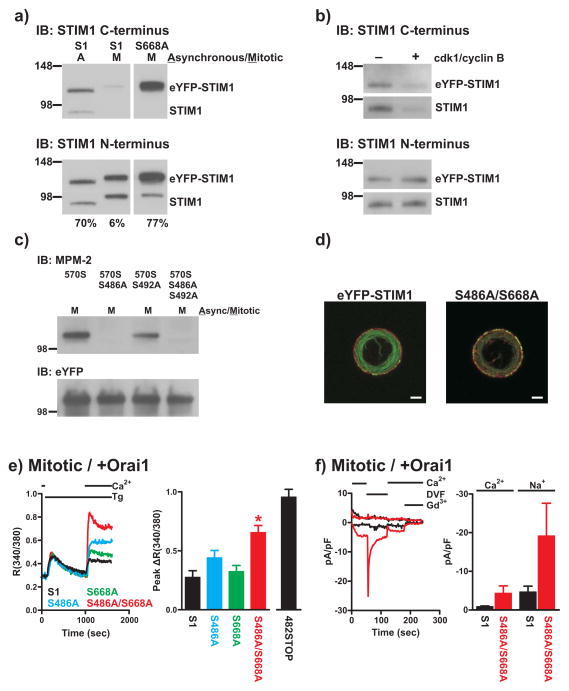
To identify individual phosphorylation sites of STIM1, eYFP-STIM1 immunoprecipitated from asynchronous or mitotic HEK293 cell lysates was subjected to mass spectrometry analysis (Supplemental Table 1). Phosphorylated S668 was identified in the mitotic but not asynchronous sample, suggesting mitosis-specific phosphorylation of S668. Phospho-S492 and phospho-S553 were also identified in the mitotic sample, but unfortunately peptides containing these residues were not recovered from the asynchronous sample. Some sites were constitutively phosphorylated (S575, S620/621), while others became dephosphorylated during mitosis (S602, S608). Thus, STIM1 phosphorylation in mitosis is complex, involving both phosphorylation and dephosphorylation, as well as constitutive phosphorylation.
From mass spectrometry analysis S668 was the strongest candidate of mitosis-specific phosphorylation. Additionally, we found that an anti-STIM1 antibody that recognizes amino acids 654 to 677 detected STIM1 in asynchronous but not mitotic samples. However, eYFP-STIM1 with S668 mutated to alanine (S668A) was recognized from mitotic lysates as effectively as eYFP-STIM1 from asynchronous lysates (Figure 5a), suggesting that phosphorylation at S668 significantly reduces the affinity of this antibody for STIM1. S668 resides within a consensus sequence for phosphorylation by cyclin-dependent kinase 1 (Cdk1), which consists of S/T-P-X-R/K. Using the STIM1 antibody to detect phosphorylation of S668, we found that both endogenous STIM1 and eYFP-STIM1 are directly phosphorylated by Cdk1 at S668 in vitro (Figure 5b).
Figure 5. Phosphorylation of S486 and S668 contributes to SOCE suppression during mitosis.
a) Total lysates from asynchronous or nocodazole-arrested mitotic HeLa cells expressing eYFP-STIM1 (S1) or S668A were analyzed by Western blot with the anti-STIM1 C-terminus antibody from ProSci (upper panel). The blot was stripped and re-probed with an anti-STIM1 N-terminus antibody (lower panel). Expressed eYFP-tagged and endogenous STIM1 bands are visible. Note that the mitotic eYFP-STIM1 band is only faintly visible with the ProSci antibody and the endogenous mitotic STIM1 band is not visible at all, despite comparable protein amounts with the asynchronous samples (based on the STIM1 N-terminus blot). The values below the blots represent the intensity of the ProSci eYFP-STIM1 or S668A bands as a percentage of the STIM1 N-terminus bands. All lanes were from the same blot, but additional lanes between the eYFP-STIM1 and S668A lanes were removed. Full scans of these blots are shown in Supplemental Figure 6e. b) Crude membrane fractions from wildtype or eYFP-STIM1 expressing HEK293 cells were treated with (+) or without (−) recombinant Cdk1/cyclin B and analyzed by Western blot with the ProSci STIM1 C-terminus antibody (upper panel). Note the diminished immunoreactivity of STIM1 from Cdk1/cyclin B-treated samples, indicative of STIM1 phosphorylation at S668. Blots were stripped and reprobed with the N-terminus STIM1 antibody to reveal total STIM1 in each sample. Full scans of these blots are shown in Supplemental Figure 6f. c) 570STOP containing the indicated S486A or S492A mutations were immunoprecipitated from nocodazole-arrested mitotic HEK293 cell lysates with an anti-eYFP antibody and analyzed by Western blot with the MPM-2 antibody (upper panel). The blot was then stripped and re-probed with the anti-eYFP antibody to reveal total protein amounts (lower panel). Full scans of these blots are shown in Supplemental Figure 6g. d) Confocal images of mitotic, thapsigargin-treated cells expressing eYFP-STIM1 and CFP-Orai1 (left) or S486A/S668A and CFP-Orai1 (right). eYFP-STIM1 and S486A/S668A are green and CFP-Orai1 is red; scalebar is 5 μm. e) SOCE responses were measured upon 1.0 mM Ca2+ restoration in nocodazole-arrested mitotic HEK293 cells co-expressing Orai1 with eYFP-STIM1 (black trace), S486A (blue trace), S668A (green trace), or S486A/S668A (red trace). Each trace represents the averaged response of 20–30 cells from a single experiment. For the bar graph in the right panel, the peak increase in fluorescence ratio above baseline following Ca2+ add-back was calculated and averaged for each cell from experiments carried out as described. For comparison, data with 482STOP from Figure 4b are also shown. eYFP-STIM1: n = 78 cells, 3 coverslips; S486A: n = 87 cells, 3 coverslips; S668A: n = 89 cells, 3 coverslips; S486A/S668A: n = 95 cells, 3 coverslips. ‘*’ indicates statistically significant difference compared to eYFP-STIM1 (one-way ANOVA followed by Tukey-Kramer; p < 0.05). Error bars represent S.E.M. f) Whole-cell Icrac currents were measured in mitotic eYFP-STIM1+Orai1 (black) and S486A/S668A+Orai1 cells (red). The whole-cell condition was achieved in the presence of 2.0 mM external Ca2+, followed by switch to a divalent-free (DVF) external solution at 60 seconds. External Ca2+ was restored at 120 seconds, and 5 μM Gd3+ was added at 180 seconds to demonstrate full inhibition of Icrac currents. Current densities measured at −100 and +100 mV are plotted, and each trace represents the response of a single cell. The bargraphs show average peak leak-subtracted Ca2+ and Na+ currents (including non-responding cells). Mitotic eYFP-STIM1+Orai1: n = 12 cells; mitotic S486A/S668A+Orai1: n = 13 cells. Error bars represent S.E.M.
S668A was still recognized by MPM-2 (not shown), indicating the presence of additional phosphorylation sites. S492 was also a potential MPM-2 site that was identified by mass spectrometry; however, the mass spectrometry data were ambiguous as to whether S492 is phosphorylated specifically during mitosis. Thus, we truncated eYFP-STIM1 at amino acid 570 (570STOP), which retains potential MPM-2 sites only at S486 and S492. 570STOP failed to support SOCE or to show membrane-associated puncta formation in mitotic cells (not shown). 570STOP immunoprecipitated from mitotic HeLa cells was recognized by MPM-2, but recognition was significantly reduced compared to mitotic eYFP-STIM1 (Figure 5c). We next mutated S486, S492, or both in 570STOP to alanine (570STOP_S486A, 570STOP_S492A, or 570STOP_S486A/S492A respectively). We were surprised to find that MPM-2 was completely unable to recognize mitotic 570STOP_S486A or 570STOP_S486A/S492A, whereas recognition of 570STOP_S492A was unaffected (Figure 5c). Thus, phosphorylation of S486 but not S492 contributes to MPM-2 recognition of mitotic eYFP-STIM1. The reason that this site was not identified by mass spectrometry, and whether S492 is phosphorylated specifically in mitosis but not recognized by MPM-2 are unclear.
We next tested the roles of the two identified sites in suppression of SOCE. Constructs carrying either single site mutation (S668A or S486A) failed to significantly rescue SOCE in mitotic cells when co-expressed with Orai1 (Figure 5e). However, mitotic cells co-expressing Orai1 and eYFP-STIM1 with both S486 and S668 mutated to alanine (S486A/S668A) exhibited SOCE responses significantly greater than mitotic eYFP-STIM1+Orai1 cells (Figure 5e). Puncta formation of S486A/S668A in mitotic cells was also more extensive than that of eYFP-STIM1 (Figure 5d and Supplemental Figure 6). Six of 13 mitotic S486A/S668A+Orai1 cells showed Icrac of 1 pA/pF or greater compared to only 2 of 12 mitotic eYFP-STIM1+Orai1 cells (Figure 5F). These data collectively suggest that phosphorylation of both S486 and S668 contributes to SOCE suppression in mitosis. We also mutated S486 and S668 to potentially phosphorylation-mimicking aspartic acids (S486D/S668D); however, SOCE responses and puncta formation were unaffected when co-expressed with Orai1 (not shown). It is therefore possible that although phosphorylation of these sites clearly contributes to inhibition of SOCE, it may not be sufficient for full suppression. However, aspartic acids do not always mimic phosphorylation. The rescue of SOCE by S486A/S668A was not as great as that by 482STOP, further suggesting that other phosphorylation sites contribute. Because mass spectrometry indicated that S602 and S608 are phosphorylated during interphase but dephosphorylated in mitosis, we considered that dephosphorylation of these sites may be involved. However, mutation of these sites to alanine failed to suppress SOCE in interphase cells (not shown). In conclusion, our data clearly indicate a role of S486 and S668 phosphorylation in SOCE suppression in mitosis, but other determinants including additional phosphorylation sites are likely involved.
The mechanism by which STIM1 phosphorylation prevents store depletion-induced rearrangement into near-plasma membrane puncta is not clear. The fact that the large truncation of 482STOP supports SOCE, albeit less efficiently, suggests that the far C-terminus of STIM1 may not be involved in direct activation of Orai, but rather serves as a regulatory region that modulates STIM1 function depending on its state of phosphorylation. This is consistent with recent publications documenting a specific Orai interacting domain upstream of amino acid 482 32–35. Regulation might result from the addition of negative charges preventing oligomerization due to electrostatic repulsion. STIM1 oligomerization precedes the movement of STIM1 toward the plasma membrane upon Ca2+ store depletion,36 and therefore prevention of oligomerization could underlie the lack of STIM1 rearrangement. Phosphorylation may also interfere with interaction of STIM1 with unknown determinants that target STIM1 into near-plasma membrane punctae. Intriguingly, S668 is near the poly-lysine region of STIM1 that has been suggested to interact with plasma membrane phospholipids.36
It is also possible that STIM1 phosphorylation may contribute to functions other than SOCE suppression. For example, phosphorylation of STIM1 may underlie dissociation of STIM1 from microtubules during mitosis. Despite the fact that dissociation of STIM1 from microtubules does not appear to be essential for SOCE regulation, it may be necessary to prevent STIM1 from interfering with the function of the mitotic spindle. STIM1 interacts with the microtubule binding protein EB-1, and this interaction was mapped to a region of STIM1 between amino acids 392 and 652,24 which includes several STIM1 phosphorylation sites. Therefore, it is possible that phosphorylation regulates the interaction of STIM1 with EB-1, preventing its localization to the mitotic spindle.
An important issue is the physiological reason for phosphorylation of STIM1 during mitosis. While it seems most likely that suppression of SOCE is important, this is not necessarily the only reason. It is also possible that STIM1 is phosphorylated in order to carry out some novel function during mitosis, and loss of its ability to activate Orai may be a secondary consequence. If this were the case, then phosphorylation of STIM2 might partially fulfill this function. This might explain why mutations that partially rescued SOCE during mitosis did not prevent cell division. It is also possible that phosphorylation of specific sites prevents their interaction with other cellular components, and deletion or mutation of these sites might accomplish the same thing, again resulting in a protein that is not detrimental to mitosis.
Despite these considerations, we did carry out a quantitative analysis of the growth rate of HEK293 cells expressing 482STOP. However, the analysis was complicated by the fact that overexpression of full length STIM1+Orai1 significantly slowed growth; the reason for this effect is not clear but likely relates to largely exaggerated SOCE responses. Despite this, the number of eYFP-STIM1+CFP-Orai1 cells following three days of growth was increased 1.79 ± .12 fold, but this value was only 1.25 ± .13 for 482STOP+CFP-Orai1 cells (p = 0.004; paired t-test; n = 7), suggesting a slower proliferation rate of 482STOP+CFP-Orai1 cells. Cell cycle analysis revealed that on the third day of growth, 3.02 ± 1.13 % of the population of eYFP-STIM1+CFP-Orai1 cells were at the G2/M stage compared to 7.07 ± .85 % for 482STOP+CFP-Orai1 cells (p = 0.04; paired t-test; n = 3). This increase in the proportion of 482STOP cells at G2/M is consistent with a delay in mitosis, possibly due to a failure to fully suppress SOCE. Although these changes are relatively small, it should be noted that cells in culture may only infrequently encounter store depleting stimuli during mitosis. In contrast, cells in their physiological environments are continuously inundated with potentially store depleting agonists, and suppression of SOCE during mitosis may be an adaptive response that insulates mitotic cells from harmful Ca2+ influx.
METHODS
Reagents and plasmids
Nocodazole was from Calbiochem, and thapsigargin was from Alexis Biochemicals. STIM1 antibodies were from ProSci (C-terminus; Product 4119) and Sigma (N-terminus; Product S6072). The Orai1 antibody was from Sigma. The eYFP, α-tubulin, and β-actin antibodies were from AbCam. The siRNA targeted to human Orai1 had the sequence cccuucggccugaucuuuaucgucu and the control siRNA was SiControl from Dharmacon. cDNAs encoding eYFP-tagged human STIM1 (eYFP-STIM1) and eYFP-tagged human STIM2 (eYFP-STIM2) were obtained from Dr. Tobias Meyer, Stanford University, and mutations of eYFP-STIM1 were generated using the QuikChange Site-Directed Mutagenesis kit (Stragagene). Untagged human Orai1 was purchased from Origene and CFP-tagged human Orai1 was constructed as previously described.37
Cell culture and transfections
HEK293 and HeLa cells (both from ATCC) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and 2 mM glutamine in a humidified CO2 incubator at 37° C. Transient cDNA transfections were carried out on cells plated to 90% confluency using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions, and transfected cells were used in experiments 48–72 hours later. siRNA transfections were similarly carried out using the HiPerFect reagent (Stratagene), and transfected cells were used 48 hours later.
Most immunoprecipitation analyses of STIM1 phosphorylation were carried out using HeLa cells because we found that non-mitotic HeLa cells remained well attached to the culture dish during collection of mitotic cells, allowing for collection of relatively pure mitotic samples. In contrast, it was more difficult to obtain mitotic HEK293 cell samples for biochemical analyses without significant contamination of non-mitotic cells. Most of our functional analyses employed HEK293 cells to take advantage of more efficient expression of co-transfected proteins.
Intracellular Ca2+ measurements
Intracellular Ca2+ measurements were carried out as previously described.38 Briefly, cells were loaded with the Ca2+ sensitive dye fura-5F and were maintained in HEPES-buffered saline solution (HBSS; in mM: 120 NaCl, 5.4 KCl, 0.8 MgCl2, 11 glucose, and 20 HEPES, pH 7.4) at room temperature throughout the course of the experiments. Fluorescence values at excitation wavelengths of 340 and 380 nm were measured consecutively at 6 second intervals for 20–30 individual cells on a single coverslip using a microscope-based digital imaging system (Intracellular Imaging), and data representing relative intracellular Ca2+ changes are plotted as 340/380 fluorescence ratios. Cells expressing eYFP-STIM1 or mutants thereof were selected at the beginning of the experiments based on eYFP fluorescence when excited at 477 nm.
Confocal microscopy
Confocal microscopy was carried out using a Zeiss LSM 510 laser scanning system and a 63X oil-immersion lens (NA 1.4). For the mitotic image shown in Figure 4a, a stack of 128 images was deconvolved using Huygens software based on a theoretical point-spread function. For fixation, cells plated on LabTek chamber slides (Nalge Nunc) were rinsed in phosphate buffered saline (PBS; in mM: 137 NaCl, 2.68 KCl, 1.47 KH2PO4, 14.9 Na2HPO4, pH 7.4), fixed for 1 minute in 0.25% gluteraldehyde, lysed for 1 minute in Karsenti’s extraction buffer (in mM: 80 Pipes, 1.0 MgSO4, 5.0 EGTA, 0.5% Triton X- 100), post-fixed in gluteraldehyde for 10 minutes, and quenched in 1 mg/ml Na- borohybride for 10 minutes. Fixed cells were incubated with primary antibodies diluted in PBS containing 0.1% Tween-20 and 3.0 mM NaN3 (PBS-TA) for 1 hour at 37° C, washed in PBS-TA, and incubated with secondary antibody (Alexa Fluor 633 goat anti-mouse; Invitrogen). Following further washes, cells were mounted using ProLong Gold with DAPI reagent (Invitrogen). For live cells, cells were maintained in HBSS at room temperature throughout the course of the experiments.
Electrophysiology
Whole-cell currents were measured in cells bathed in HBSS at room temperature using the patch-clamp technique. The intracellular pipette solution contained in mM 145 cesium methanesulfonate, 20 BAPTA, 10 HEPES, and 8 MgCl2 (pH 7.2 with CsOH). The pipette solution also contained 25 μM inositol 1,4,5-trisphosphate (IP3; Sigma) to rapidly deplete intracellular Ca2+ stores upon break-in. Voltage ramps (+100 to −100 mV) of 250 ms were recorded every 2 seconds from a holding potential of 0 mV, and currents were acquired using pCLAMP 10 software (Axon Instruments). All currents were normalized based on cell capacitance, and leak currents were taken immediately after break-in before Icrac activation. Solutions were applied using a gravity-based multibarrel focal perfusion system (Perfusion Pencil, Automate Scientific).
Immunoprecipitation and Western blotting
Cells were lysed in RIPA buffer (in mM: 50 Tris HCl, 150 NaCl, 1 EDTA, 1 phenylmethylsulfonyl fluoride, 1% v/v NP-40, and 0.25% w/v sodium deoxycholate) supplemented with 1X Complete Mini Protease Inhibitor (Roche Applied Sciences) and 1X Halt Phosphatase Inhibitor Cocktail (Thermo Scientific). Protein concentrations were determined using the DC protein assay kit (BioRad). For immunoprecipitations, lysates containing equal protein amounts were pre-cleared for 1 hour with Protein A/G beads (Thermo Scientific), incubated with primary antibody overnight, and incubated with Protein A/G beads for an additional 4 hours, all at 4° C. Beads were then washed 3 times with RIPA buffer, and proteins were eluted in Laemmli sample buffer containing 5% β-mercaptoethanol for 5 minutes at 95° C. For Western blotting, protein lysates diluted in Laemmli sample buffer containing 5% β-mercaptoethanol or eluates from immunoprecipitations were electrophoresed in 6% or 10% gels and transferred to PVDF membranes. All antibody dilutions and washes were carried out in Tris-buffered saline (TBS; in mM: 137 NaCl, 19 Tris HCl, 2.7 KCl, pH 7.4) containing 0.1% Tween-20 (TBS-T). Membranes were blocked in 3% BSA in TBS-T for 1 hour at room temperature, incubated with primary antibodies overnight at 4° C, and incubated with secondary antibodies (horseradish peroxidase-linked anti-mouse or anti-rabbit; GE Healthcare) for 45 minutes at room temperature. Blots were developed with ECL Plus (GE Healthcare) and exposed to film. Stripping was carried out using Restore reagent (Thermo Scientific). For quantification of band intensities, films were scanned and analyzed by densitometry using Photoshop software.
In Vitro Cdk1 Kinase Assay
Crude membrane fractions were prepared by scraping HEK293 cells into hypotonic buffer (in mM: 10 Tris-HCL, 10 NaCl, 1.5 MgCl2, 1 phenylmethylsulfonyl fluoride, pH 7.5) containing 1X Complete EDTA-free Protease Inhibitor (Roche) followed by homogenization in a Dounce homogenizer. Intact cells and nuclei were removed by centrifugation at 1,000 g for 5 min, and the supernatant was then centrifuged at 25,000 g for 30 min. Membrane pellets were resuspended in Cdk1 kinase buffer (in mM: 25 Tris-HCl, 10 MgCl2, 5 β-glycerophosphate, 0.1 Na3VO4, 2 dithiothreitol, 0.2 ATP, pH 7.5) by sonication. Recombinant Cdk1/cyclin B (Cell Signaling Technology) was added at a concentration of 200 ng per 100 μl reaction volume, and reactions were incubated at 37 °C overnight. Samples were then processed for Western blotting.
Mass Spectrometry
In-gel digestion with either trypsin or GluC, nanoLC-ESI-MS/MS, automated database searching, and manual spectral interpretation were performed essentially as previously described.39 In addition to traditional collision induced dissociation, electron transfer dissociation (ETD) was also employed for MS/MS experiments. ETD settings included the use of fluoranthine as the electron donor with negative ion source settings that included a 150 eV ionization energy and a 100 msec accumulation time. To enrich for phosphopeptides, metal oxide affinity chromatography was performed using TiO2 tips (Glygen Corp.) using essentially the manufacturer’s recommended protocol.
Proliferation Rate and Cell Cycle Analysis
For analysis of proliferation rates, equal numbers of eYFP-STIM1 or 482STOP and CFP-Orai1 co-transfected cells were plated, and on each day for three days cells were trypsinized and counted using a hemocytometer. The same populations of cells were then analyzed using a LSR II flow cytometer and FACSDiva software (BD Biosciences) to determine the proportion of CFP and eYFP double positive cells. eYFP fluorescence was determined by excitation with a 488 nm laser and a 530/30 nm emission filter, and CFP by excitation with a 405 nm laser and a 525/50 nm emission filter. Gates for eYFP and CFP positive cells were established using untransfected cells. A total of 10,000 viable cells were analyzed per sample, and the proportion of double positive cells was multiplied by the total number of cells obtained by counting to determine the total number of double positive cells per sample. The total number of double positive cells on day 3 was divided by that on day 1 to obtain the proliferation rate. For cell cycle analysis, trypsinized cells were fixed in 70% ethanol at 4 °C overnight, pelleted, and re-suspended in propidium iodide (PI) solution (20 μg/ml PI, 10 Units/ml Rnase in PBS) for 20 min. Cells were then analyzed by flow cytometry to initially identify CFP and YFP positive cells as described, followed by cell cycle analysis. DNA content was determined using PI fluorescence with an excitation at 488 nm and 575/26 nm emission on a double discrimination dot plot based on PI area versus width (to exclude cell aggregates). Data were then modeled using Modfit LT software (Verity Software House) to obtain proportions of cells at specific cell cycle stages.
Mitotic arrest
Cells were arrested in mitosis by treating logarithmic phase populations of asynchronous cells with 1.67 μM nocodazole for 12–16 hours. This resulted in approximately 70–80% mitotic cells based on spherical morphology.23 For live-cell experiments, unless otherwise indicated, mitotic cells were then detached by gentle aspiration and plated onto poly-L-lysine (0.01%; Sigma) coated coverslips for 30 minutes. All solutions in these experiments contained 1.67 μM nocodazole to maintain mitotic arrest. For experiments with interphase cells, unless otherwise indicated, flat, well-spread cells were selected from populations of asynchronous cells. For biochemical analyses, nocodazole-arrested mitotic cells were detached in PBS, collected by centrifugation, and lysed in RIPA buffer.
Statistical analyses
Statistical analyses were carried out using GraphPad Prism Software.
Supplementary Material
Acknowledgments
We thank Jeff Tucker and Holly Rutledge for assistance with confocal microscopy and Carl Bortner and Maria Sifre for help with flow cytometry. Dr. Carmen Williams and Dr. David Miller reviewed the manuscript and offered helpful suggestions. This work was supported by the Intramural Program, National Institute of Environmental Health Sciences, National Institutes of Health.
Reference List
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 4.Ng SW, Di Capite J, Singaravelu K, Parekh AB. Sustained Activation of the Tyrosine Kinase Syk by Antigen in Mast Cells Requires Local Ca2+ Influx through Ca2+ Release-activated Ca2+ Channels. J Biol Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 5.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586:3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–355. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 7.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 10.Vig M, et al. CRACM1 Is a Plasma Membrane Protein Essential for Store-Operated Ca2+ Entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. The Journal of Cell Biology. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 15.Putney JW., Jr New molecular players in capacitative Ca2+ entry. J Cell Sci. 2007;120:1959–1965. doi: 10.1242/jcs.03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston SF, Sha’afi RI, Berlin RD. Regulation of Ca2+ influx during mitosis: Ca2+ influx and depletion of intracellular Ca2+ stores are coupled in interphase but not mitosis. Cell Regul. 1991;2:915–925. doi: 10.1091/mbc.2.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preston GA, Barrett JC, Biermann JA, Murphy E. Effects of alterations in calcium homeostasis on apoptosis during neoplastic progression. Cancer Res. 1997;57:537–542. [PubMed] [Google Scholar]
- 18.Volpi M, Berlin RD. Intracellular elevations of free calcium induced by activation of histamine H1 receptors in interphase and mitotic HeLa cells: hormone signal transduction is altered during mitosis. J Cell Biol. 1988;107:2533–2539. doi: 10.1083/jcb.107.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tani D, Monteilh-Zoller MK, Fleig A, Penner R. Cell cycle-dependent regulation of store-operated I(CRAC) and Mg2+-nucleotide-regulated MagNuM (TRPM7) currents. Cell Calcium. 2007;41:249–260. doi: 10.1016/j.ceca.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hepler PK. The role of calcium in cell division. Cell Calcium. 1994;16:322–330. doi: 10.1016/0143-4160(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 21.Whitaker M, Larman MG. Calcium and mitosis. Semin Cell Dev Biol. 2001;12:53–58. doi: 10.1006/scdb.2000.0217. [DOI] [PubMed] [Google Scholar]
- 22.Whitaker M. Calcium microdomains and cell cycle control. Cell Calcium. 2006;40:585–592. doi: 10.1016/j.ceca.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krek W, DeCaprio JA. Cell synchronization. Methods Enzymol. 1995;254:114–124. doi: 10.1016/0076-6879(95)54009-1. [DOI] [PubMed] [Google Scholar]
- 24.Grigoriev I, et al. STIM1 Is a MT-Plus-End-Tracking Protein Involved in Remodeling of the ER. Curr Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth JT, DeHaven WI, Bird GS, Putney JW., Jr Role of the microtubule cytoskeleton in the function of the store-operated Ca2+ channel activator STIM1. J Cell Sci. 2007;120:3762–3771. doi: 10.1242/jcs.015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russa AD, et al. Microtubule remodeling mediates the inhibition of store-operated calcium entry (SOCE) during mitosis in COS-7 cells. Arch Histol Cytol. 2008;71:249–263. doi: 10.1679/aohc.71.249. [DOI] [PubMed] [Google Scholar]
- 27.Manji SS, et al. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 28.Williams RT, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Che S, Weil MM, Nelman-Gonzalez M, Ashorn CL, Kuang J. MPM-2 epitope sequence is not sufficient for recognition and phosphorylation by ME kinase-H. FEBS Lett. 1997;413:417–423. doi: 10.1016/s0014-5793(97)00948-4. [DOI] [PubMed] [Google Scholar]
- 30.Ding M, Feng Y, Vandre DD. Partial characterization of the MPM-2 phosphoepitope. Exp Cell Res. 1997;231:3–13. doi: 10.1006/excr.1996.3439. [DOI] [PubMed] [Google Scholar]
- 31.Westendorf JM, Rao PN, Gerace L. Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci U S A. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009 doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muik M, et al. A cytosolic homomerization and a modulatory domain within STIM1 C-terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeHaven WI, Smyth JT, Boyles RR, Putney JW. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 38.Smyth JT, DeHaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and -independent reversal of Stim1 localization function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JH, Williams J, Cho J, Falck JR, Shears SB. Purification, sequencing, and molecular identification of a mammalian PP-InsP5 kinase that is activated when cells are exposed to hyperosmotic stress. J Biol Chem. 2007;282:30763–30775. doi: 10.1074/jbc.M704655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.