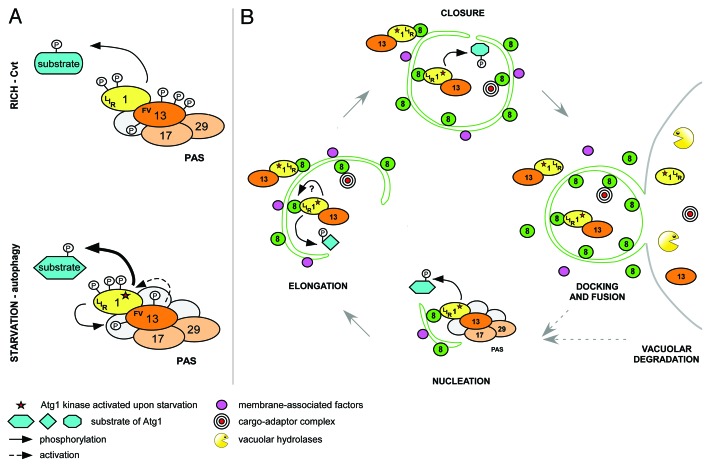
Figure 1. Model of temporal and spatial regulation of the Atg1 kinase complex. (A) Atg1 and Atg13 interact constitutively in rich and starved conditions via the FV motif. Upon starvation, phosphorylation changes on Atg1 and Atg13 activate Atg1 kinase and modulate substrate specificity by induction of conformational changes and/or recruitment of additional regulators. Atg1 and Atg13 may form a reinforcing feedback loop where Atg13 is phosphorylated in an Atg1-dependent manner to stimulate Atg1 kinase activity. (B) At the PAS, Atg1 regulates association and dissociation of Atg proteins to initiate autophagy. Activated Atg1-Atg13 complexes are then tethered to autophagosomal membranes by Atg8 via a conserved LIR motif on Atg1. This Atg1 pool promotes membrane elongation and/or closure by phosphorylation of unknown targets, or by regulating protein dynamics at the membrane. After docking and fusion of autophagosomes with the vacuole, Atg1-Atg13 complexes are degraded, modulating autophagy flux with a negative feedback mechanism.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.