Figure 1.
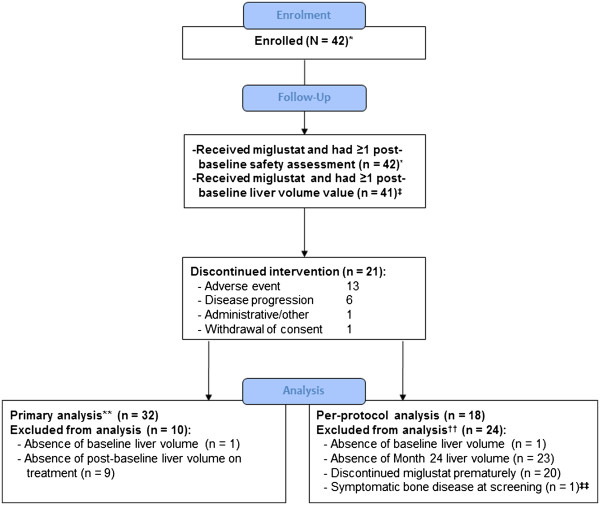
Patient disposition.*All enrolled population; ‡full analysis population; **all patients with at least one post-baseline liver assessment (≥6 months) were included in primary endpoint analysis, including 11 patients who discontinued miglustat treatment prematurely; ††some patients had more than one of the listed reasons for non-inclusion in the per-protocol analysis; ‡‡Patient had documented symptomatic bone disease at screening and represented a protocol violation. Note: secondary efficacy evaluations based on full analysis population included different numbers of patients subject to data availability.
