Figure 5. Quality of sOMV and eOMV vaccines.
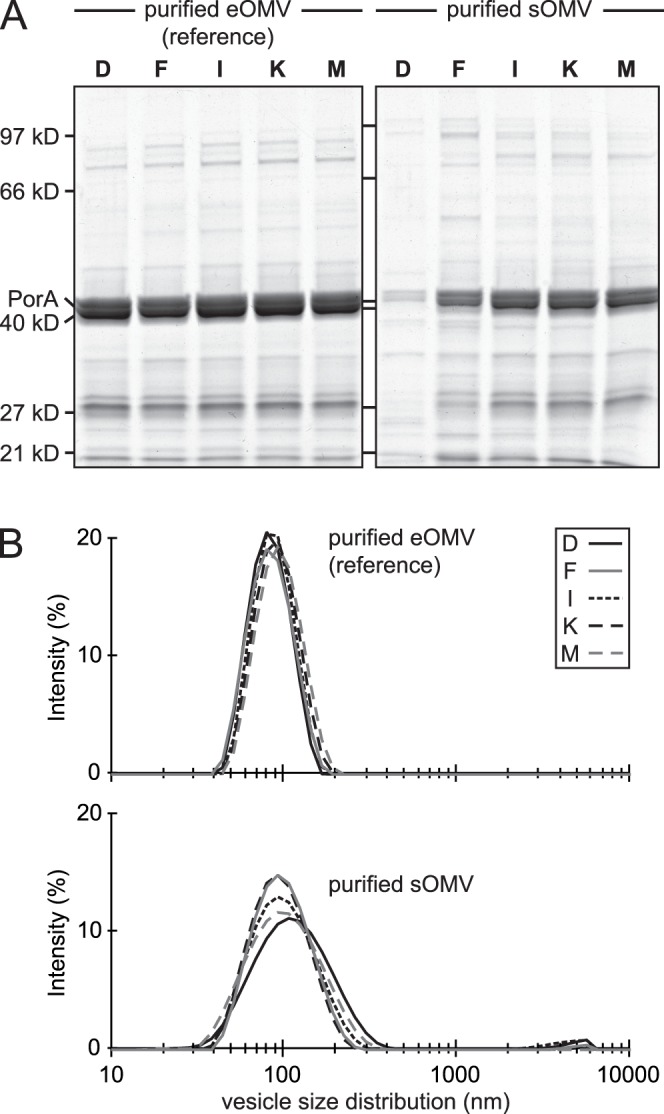
In addition to yield, quality of the sOMV and eOMV vaccines is compared. It was previously demonstrated that both vaccine types provide low toxicity and high functional immunogenicity in mice [11]. (A) Protein composition of eOMV reference vaccines is comparable to sOMV vaccines after cysteine depletion (time points I, K, M). Each lane contains 4 µg total protein, except sOMV at time points D and F (low protein concentration due to a low yield; maximal sample volume is loaded). PorA antigen (∼41 kD) has a major contribution to total protein content (>60%) in all vaccines. (B) Dynamic light scattering analysis reveals that sOMV vaccines have a slightly broader size distribution and minor aggregation compared to the eOMV references, indicating that the purification procedure is not yet fully consistent. X-axis represents vesicle size distribution (nm).
