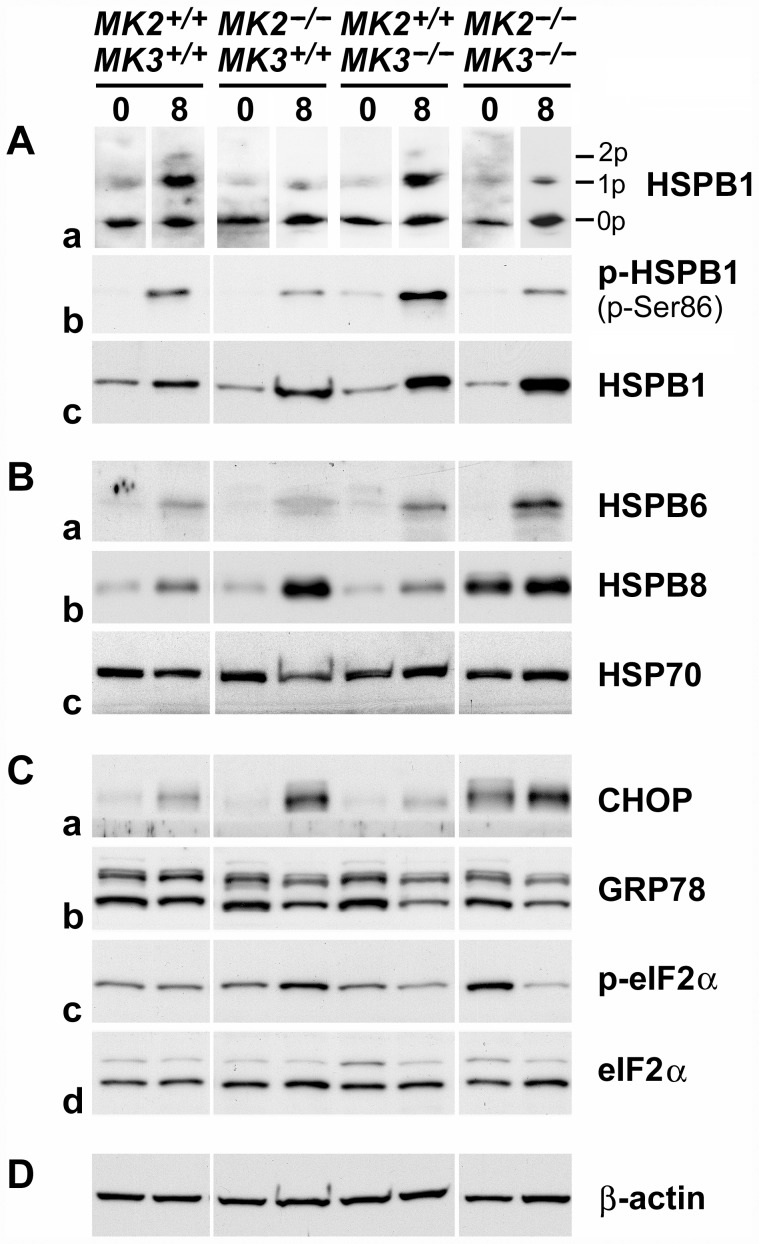
Figure 5. p38 MAPK→MK2/MK3→HSPB1 signaling and stress response in MK2/MK3 knock-out mice following AMC serum treatment.
Extracts of renal cortices were processed for IEF-PAGE (A, panel a) or SDS-PAGE (A, panels b, c; B, C, D) from untreated mice (day 0; baseline control) and AMC serum-treated mice (day 8 of treatment). (A) Phosphorylation, baseline expression and induction of HSPB1. Panel a shows the distribution of the various HSPB1 isoforms (0p, unphosphorylated; 1p, singly phosphorylated; 2p, doubly phosphorylated) within each sample. Sample loading aimed to obtain comparable overall signals, in spite of considerable differences in the total HSPB1 content among the samples (cf. panel c). Panel b shows the amounts of Ser86-phosphorylated HSPB1 (p-Ser86). Equal amounts of total protein (15 µg) were loaded onto each lane. Panel c shows baseline expression and induction of HSPB1 in response to the AMC serum. (B) Baseline expression and response to the AMC serum of the heat shock proteins, HSPB6, HSPB8, and HSP70 (panels a–c, respectively). (C) Expression or phosphorylation of indicators of the unfolded protein response, CHOP (panel a), GRP78 (panel b), and eIF2α (panels c, d), before and after AMC serum treatment. Panels c and d show phosphorylated (p-eIF2α) and total eIF2α, respectively. (D) Expression of β-actin served as a loading control. Overall, this figure demonstrates partial involvement of MK2 and MK3 in baseline expression and/or phosphorylation of a number of sHSPs and indicators of the unfolded protein response, as well as in their pathophysiological response following AMC serum treatment.