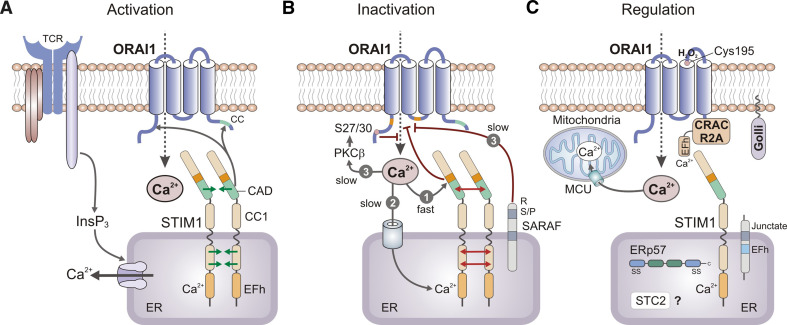
Fig. 4.
Mechanisms of CRAC channel activation, inactivation, and regulation. a CRAC channel activation. Depletion of Ca2+ from ER stores results in dissociation of Ca2+ from the EF-hand (EFh) in the N-terminus (NT) of STIM1 (and STIM2, not shown), unfolding of the STIM1-NT consisting of EFh and adjacent SAM domains and oligomerization of STIM1 molecules. Oligomerization is mediated by the interaction of multiple protein domains within STIM1 including EFh-SAM, CC1, and CAD domains. The CRAC channel activation domain (CAD, alternatively called SOAR or CCb9) in the C-terminus of STIM1 is sufficient for binding to ORAI1 and CRAC channel activation. b Fast and slow CRAC channel inactivation is mediated by distinct mechanisms. (1) Fast Ca2+-dependent CRAC channel inactivation (CDI) occurs over tens of milliseconds and is mediated by acidic residues in the STIM1-CT immediately adjacent to CAD, a calmodulin-binding domain (aa 70–87) in the N-terminus of ORAI1 and a NVHNL polypeptide in the II–III loop of ORAI1 (all indicated in orange). (2) Slow store-dependent inactivation of CRAC channels occurs over tens of seconds and is mediated by Ca2+ re-uptake into the ER via SERCA pumps, resulting in Ca2+ re-binding to the EFh in STIM1 and STIM1 de-oligomerization. (3) Slow store-independent CRAC channel inactivation also occurs over tens of seconds and is regulated by several mechanisms, including interaction of STIM1 with SARAF and potentially PKCβ-mediated phosphorylation of ORAI1 on N-terminal serine residues S27 and S30 (SF, unpublished). c Regulation of CRAC channel function by several modulatory proteins. CRACR2A forms a ternary complex with ORAI1 and STIM1 to promote puncta formation and CRAC channel activation. Junctate is a single-pass transmembrane protein in the ER that facilitates recruitment of STIM1 (and ORAI1) to ER-PM junctions. Golli is a cytoplasmic protein that is recruited to the PM in a myristoylation-dependent manner and inhibits CRAC channel function. Stanniocalcin 2 (STC2) and the ER-resident oxidoreductase ERp57 bind to STIM1 and inhibit SOCE, but the underlying mechanisms are not well understood. ORAI1 function can be directly modulated by reactive oxygen species (ROS) via redox regulation of a reactive cysteine (C195) in its III–IV loop. Finally, Ca2+ uptake into mitochondria located close the plasma membrane via the mitochondrial uniporter (MCU) prevents Ca2+-dependent CRAC channel inactivation by curtailing a rise in local Ca2+ concentrations. CAD CRAC channel activation domain, CC coiled-coil, EFh EF-hand, SAM sterile-alpha motif, SARAF SOCE-associated regulatory factor, SERCA sarcoplasmic/endoplasmic reticulum Ca2+ ATPase