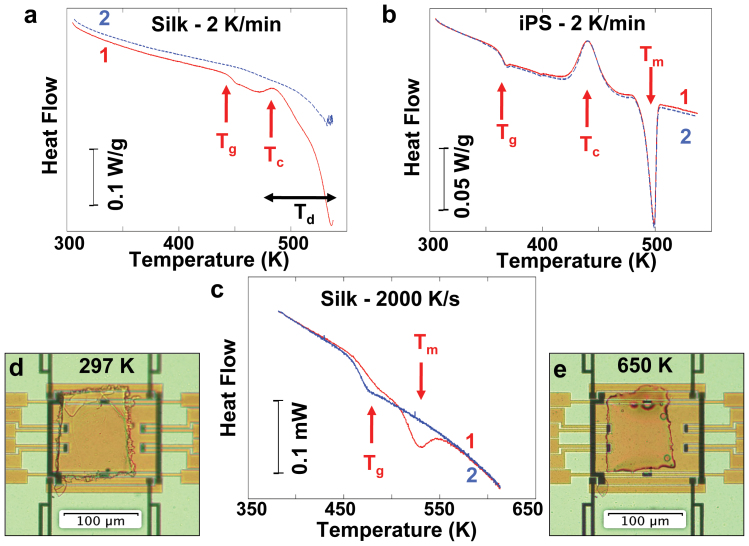
Figure 2. Comparison of Thermal Characteristics of Silk and Synthetic Polymer at Different Heating Rates.
Observable transitions during first (1, red) and second (2, blue) heating include glass transition (Tg), crystallization exotherm (Tc), melting endotherm (Tm), and thermal degradation (Td). Endotherms are presented with downward deflection. (a) Film of reconstituted B. mori silk fibroin protein during DSC heating at 2 K/min. The sample degrades and loses mass upon first heating. The second heating scan does not retrace the first, and no glass transition or crystallization can be observed. (b) Synthetic polymer (exemplified by isotactic polystyrene, iPS) during DSC heating at 2 K/min. Sample does not degrade after heating to 538 K, and the overlapping second heating trace shows the same thermal transitions. (c) Film of reconstituted B. mori silk fibroin protein, now heated at 2,000 K/s using fast scanning chip calorimetry, shows melting of beta pleated sheet crystals. The second trace, after cooling from the melt at 2,000 K/s, confirms the non-crystalline nature of the film after beta-pleated-sheets were melted during the first scan. (d, e) Sample from (c), imaged at room temperature with unpolarized light using a 20× objective on the flat, optically transparent sensor, before (d) and after (e) fast heating to 650 K. As a result of melting the film changes shape slightly.