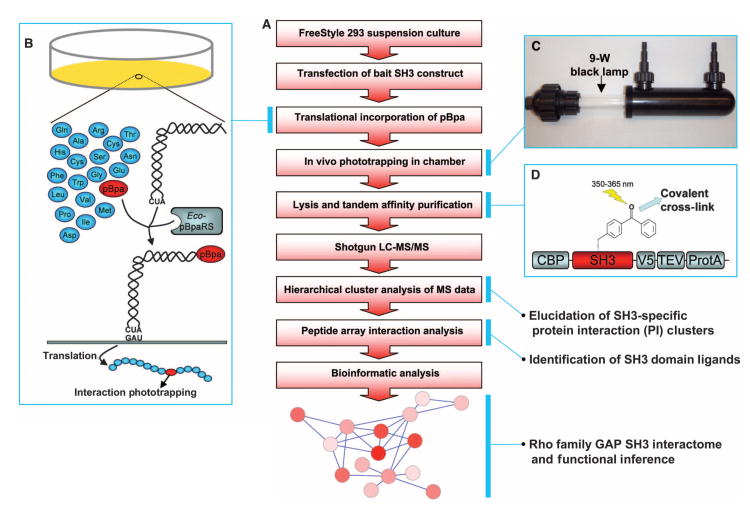
Fig. 1.
Experimental design to identify Rho family GAP protein complexes. (A) Workflow of overall approach to construct Rho family GAP interactomes. (B) Schematics for in vivo phototrapping strategy. A photo-activatable cross-linker, pBpa, is translationally incorporated into a cellular protein of interest at the site designated by an amber codon when coexpressed with a pBpa-specific tRNA synthetase and amber suppression tRNA. pBpa covalently cross-links with a binding protein when UV light is applied. (C) Modified cross-linking chamber for cells in suspension with inlet, outlet, and 9-W 350- to 365-nm light source. (D) Schematic of the SH3 domain expression construct. TAP tag (protein A, TEV protease cleavage site, and CBP) was attached to the SH3 domain for the effective isolation of SH3 domain–ligand cross-linked protein complexes. A V5 epitope was added to follow the TAP purification process.