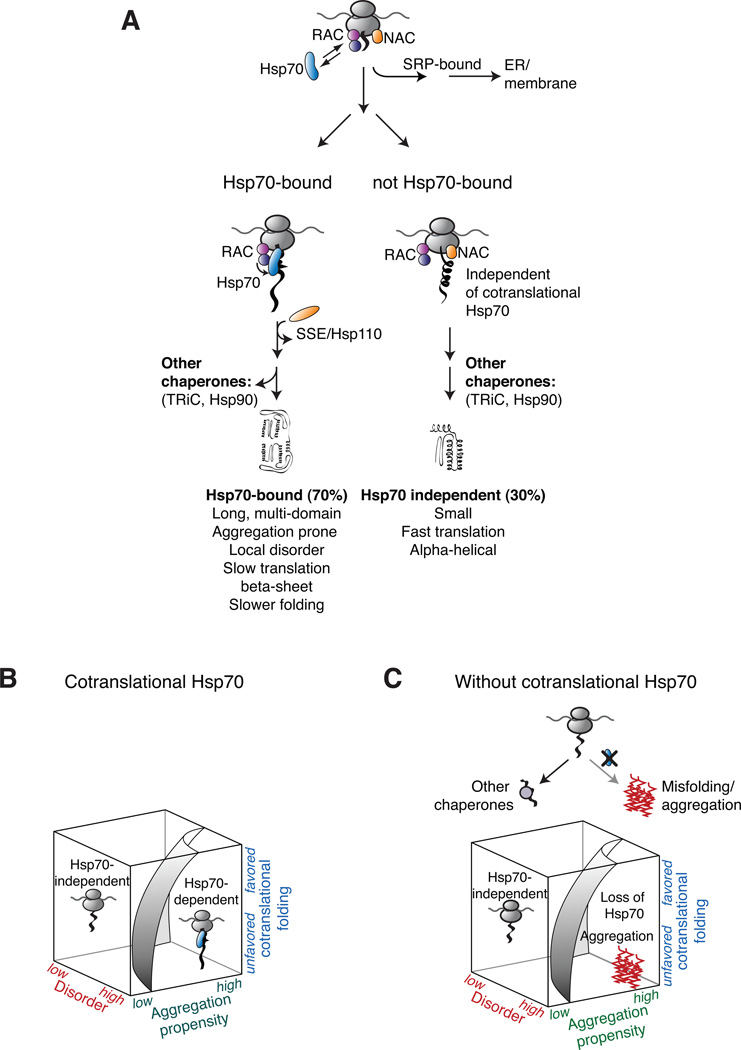
Figure 7. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis.
(A) The role of cotranslational acting Hsp70s in protecting nascent polypeptides. Hsp70 associates with approximately 70% of newly translated polypeptides with a strong enrichment of cytosolic and nuclear proteins. The cotranslational specificity of Hsp70 for its substrates is modulated by the co-chaperone RAC. Early sorting of SSB and SRP results in mutually exclusive binding to nascent chains at the ribosomes. Maturation of not Hsp70-bound proteins is likely facilitated by other chaperone like NAC. (B) Co-translationally acting Hsp70 meets the challenge of folding the eukaryotic proteome by protecting newly translated polypeptides challenged in co-translational folding. (C) Loss of co-translational acting Hsp70 leads to widespread aggregation of newly made polypeptides with properties hindered in efficient co-translational folding.