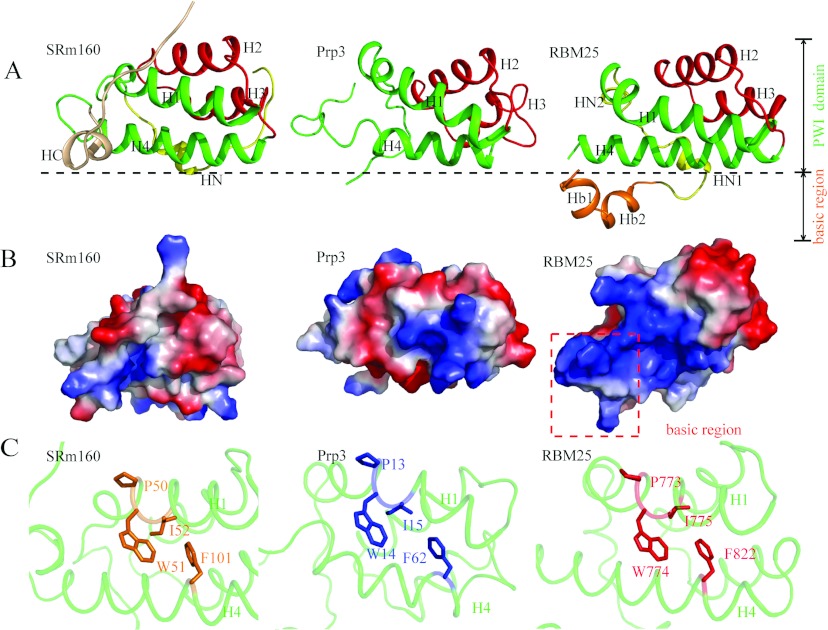
Figure 3. Structural comparison of RBM25 PWI with the SRm160 and Prp3 PWI domains.
(A) Structural comparisons of RBM25 PWI with the SRm160 and Prp3 PWI domains. Only our structure shows the structure of the flanking basic region. H1 and H4 are shown in green, H2 and H3 are shown in red, the N-elements (HN) are shown in yellow, the C-element (HC) is shown in wheat, and the flanking basic region is shown in orange. (B) Comparison of surface electrostatic potentials of RBM25 PWI domain with the SRm160 and Prp3 PWI domains. The red broken box encloses the flanking basic region. (C) The highly conserved phenylalanine residue in H4 packs against the tryptophan and isoleucine residues in the highly conserved signature Pro-Trp-Ile sequence of H1. Single-letter code is used for amino acids.