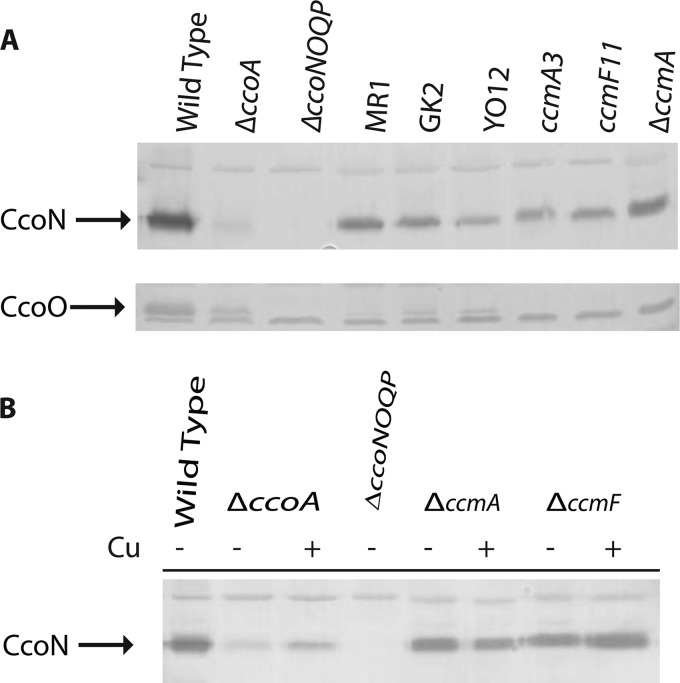
Fig 5.
Amounts of CcoN present in the membranes of various cbb3-Cox mutants and CcmA and CcmF knockout mutants of R. capsulatus. (A) Membrane fractions (∼50 μg of total protein) of the wild-type (MT1131), ΔccoA (SE8), ΔccoNOQP (GK32), MR1 (ccoO1), GK2 (ccoO2), YO12 (ΔpetABC::spe ccoP1), ccmA3 (XJ3), ccmF11 (XJ11), and ΔccoA ΔccmA (SE17) strains grown under respiratory conditions in MPYE medium in the absence of 5 μM Cu2+ were analyzed by 12.5% SDS-PAGE, and the CcoN and CcoO subunits of cbb3-Cox (indicated by arrows) were detected by specific antibodies as described in Materials and Methods. The band common to all lanes corresponds to a nonspecific protein detected by the antibodies used. (B) Membrane fractions (∼50 μg of total protein) of the wild-type (MT1131), ΔccoA (SE8), ΔccoNOQP (GK32), ΔccmA (SE20), and ΔccmF (MD12) strains grown under respiratory conditions in MPYE medium in the absence (−) or presence (+ Cu) of 5 μM Cu2+ were analyzed by 12.5% SDS-PAGE, and the CcoN subunit of cbb3-Cox (indicated by an arrow) was detected by subunit-specific antibodies as described in Materials and Methods. The bands common to all lanes correspond to nonspecific proteins detected by the antibodies used.