Fig 2.
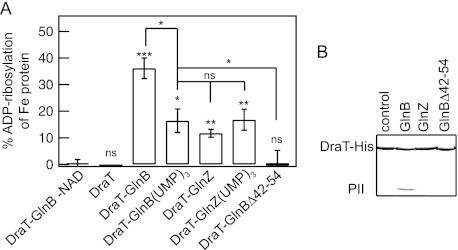
DraT activation and interaction with PII proteins. (A) DraT activity was assessed indirectly from Fe protein activity by measuring hydrogen evolution. A DraT assay was performed using A. vinelandii Fe protein as the substrate in the presence of different PII proteins to form the in vitro complexes. Reaction mixtures containing 2 mM NAD+; 1 mM MgCl2; 1 mM ADP; 100 μg Fe protein; and DraT, DraT-PII complexes formed in vitro, or copurified DraT-GlnB were incubated for 5 min at 130 rpm at 30°C. The reactions were performed using a DraT/PII trimer molar ratio of 1:2 and an Fe protein/DraT molar ratio of 21:1. For a control, the reaction was performed in the presence of either copurified complex, DraT-GlnB (1:2, as shown), or DraT in the absence of NAD+. The DraT activity was stopped by reducing NAD+ using dithionite solution. The Fe protein activity was determined by adding 500 μg of MoFe protein, and the hydrogen production was measured. The activity of the copurified DraT-GlnB complex was 130 ± 2.5 nmol ADP-ribosylated Fe protein min−1 mg DraT−1 under the same conditions, corresponding to 100% ADP-ribosylation of Fe protein. ns, nonsignificant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. The error bars represent the calculated standard deviations. (B) Complex formation between His-tagged DraT and the PII protein GlnB, GlnZ, or GlnBΔ42-54 was assessed by pulldown in the presence of 1 mM ADP. Proteins bound to Ni2+ beads were analyzed by SDS-PAGE, and the gel was stained with Coomassie blue.
