Figure 4.
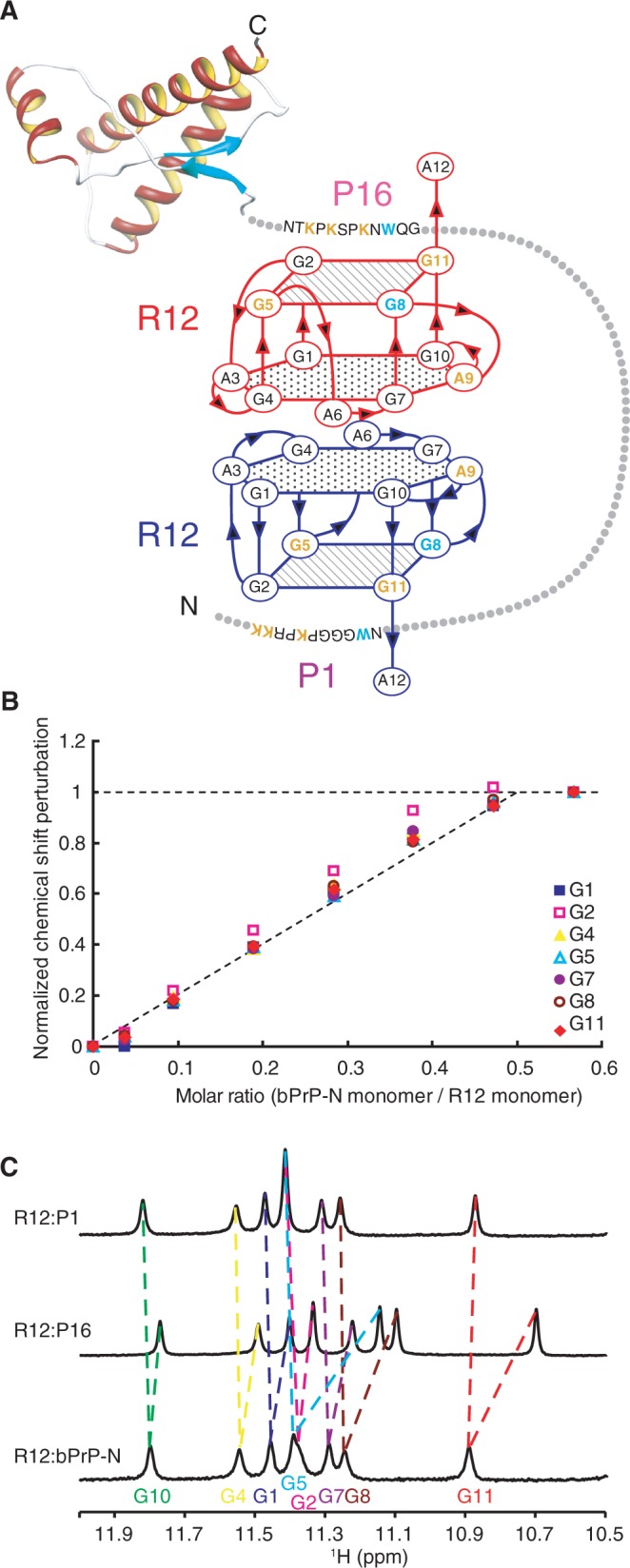
Overall architecture of tight trapping of bPrP by R12. (A) Overall architecture of the complex between bPrP and R12. The tetrad and hexad planes of R12 are indicated by a square and a hexagon, respectively. The lysine and guanosine residues involved in the electrostatic interaction are coloured orange, and the tryptophan and guanosine residues involved in the stacking interaction are coloured blue, respectively. The structure of the C-terminal region of bPrP is drawn on the basis of the coordinates under accession number 1DX0 in the Protein Data Bank (21). (B) Normalized chemical shift perturbation for each imino proton of R12 in the course of the titration with bPrP-N(25–131, 5WS). (C) Imino proton spectra of the R12:P1 complex, the R12:P16 complex and the R12:bPrP-N(25–131, 5WS) complex with the assignments.
