Figure 2.
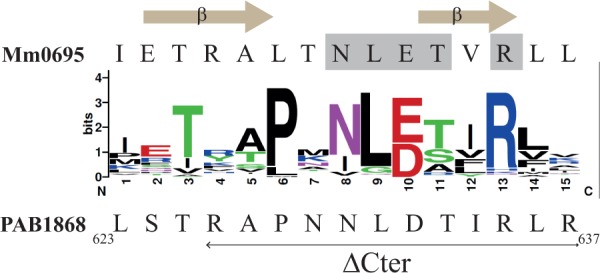
Sequence conservation of the last 16 residues of the 110 members of aCPSF1 is shown using Weblogo representation (http://weblogo.berkeley.edu). The amino acid C-terminal sequence of M. mazei Mm0695 and of P. abyssi PAB1868 (Pab-aCPSF1) are specified on the top and bottom, respectively. The residues establishing interacting hydrogen bonds in the protein–protein interface of the dimeric structure of Mm0695 (26) are highlighted in grey and the β-sheets formed by these residues are indicated. The last 12 residues that were deleted in aCPSF1ΔCter variant are indicated by a horizontal arrow under the P. abyssi sequence.
