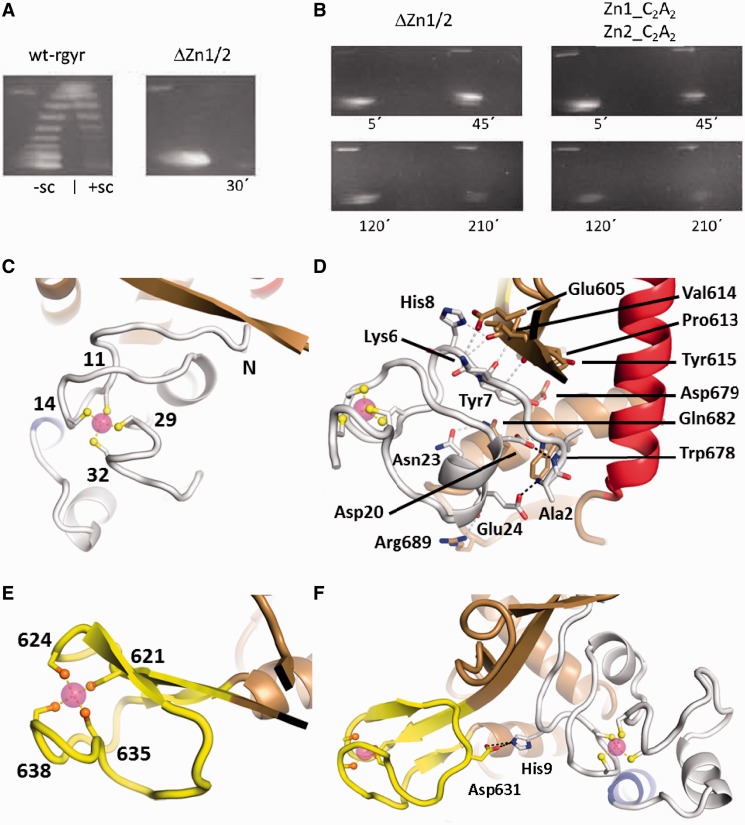
Figure 4.
The zinc fingers and their interactions. (A) Deletion of the zinc fingers abolishes supercoiling activity of reverse gyrase. Left panel: distribution of topoisomers generated by wild-type reverse gyrase (wt-rgyr) after 30 min and separated by 2D agarose gel electrophoresis. Negatively and positively supercoiled topoisomers are indicated as −sc and +sc, respectively. Right panel: deletion of the zinc fingers (ΔZn1/2) abolishes positive supercoiling and leads to almost no relaxation. (B) Both deletion of zinc fingers and mutation of individual cysteine residues in the zinc fingers impair supercoiling activities. Left panel: even after 3.5 h the zinc finger deletion construct displays no residual supercoiling activities. Right panel: mutation of two cysteine residues to alanine in both zinc fingers is sufficient for abolishment of supercoiling activities. For constructs, see ‘Materials and Methods’ section. (C) Structure of the N-terminal zinc finger. Zinc (magenta sphere) is coordinated by four cysteine residues (labeled) in an approximately tetrahedral geometry. (D) Possible hydrogen bonds between the N-terminal zinc finger and the Toprim domain are drawn as gray dashed lines. Two more hydrogen bonds in black show how the N-terminus is fixed by zinc finger residues Asp20 and Glu24. (E) The C-terminal zinc finger forms a zinc ribbon that is connected to the Toprim domain via a β-sheet. (F) Single interaction between the zinc fingers via a charged hydrogen bond.