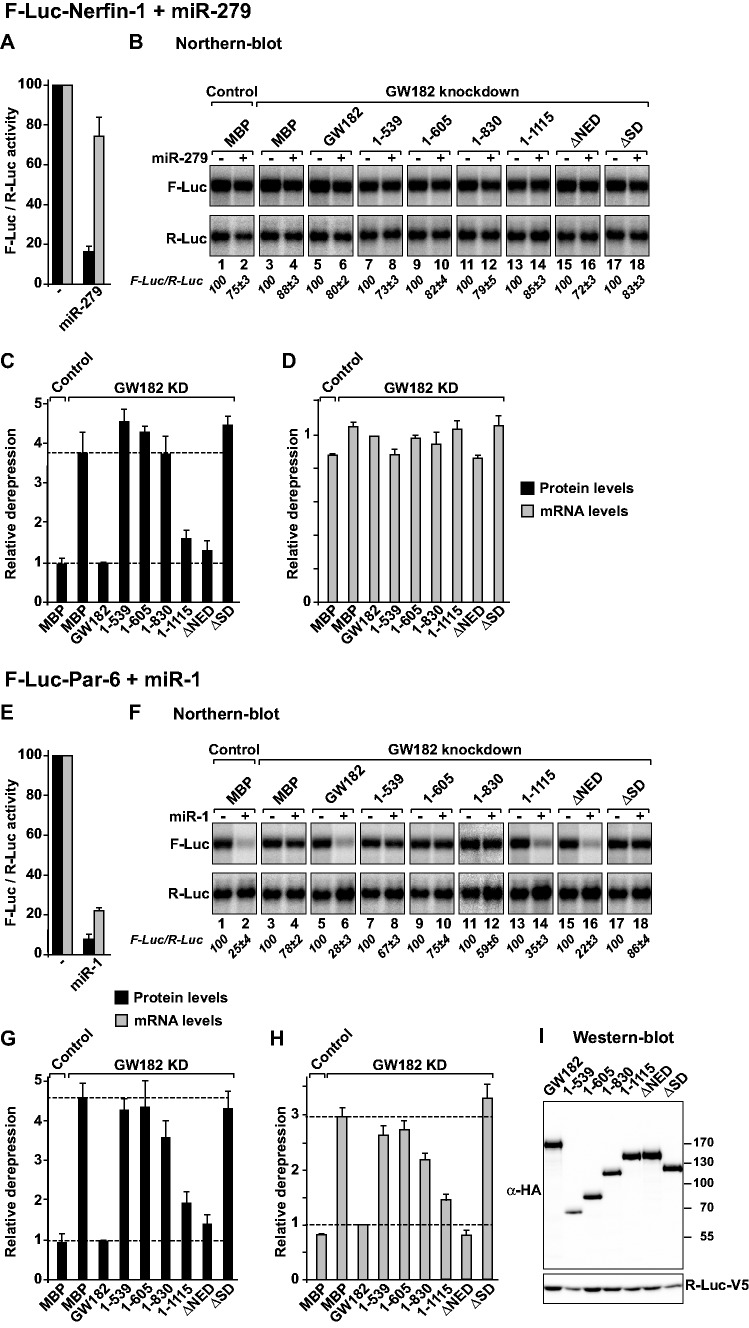
Figure 3.
The Drosophila melanogaster GW182 SD is generally required for miRNA-mediated translational repression and target degradation. (A–I) Control S2 cells (treated with glutathion S-transferase (GST) dsRNA) or cells depleted of endogenous GW182 were transfected with a mixture of three plasmids: one expressing the indicated F-Luc reporters; a second expressing miRNA primary transcripts or the corresponding empty vector (−) and a third expressing Renilla luciferase (R-Luc). Plasmids encoding HA-GW182 (wild-type or deletion mutants) or HA-MBP (negative control) were included in the transfection mixtures as indicated. For each condition, firefly luciferase activities and mRNA levels were normalized to those of the Renilla luciferase transfection control and set at 100% in cells transfected with the empty vector (i.e. in the absence of the miRNAs). (A and E) Normalized firefly luciferase activities and mRNA levels in the absence or presence of miRNAs in control cells (i.e. cells treated with GFP dsRNA and transfected with a plasmid expressing MBP). (B and F) Northern blot analysis of representative RNA samples. Numbers in italics below the panels indicate the levels of the F-Luc reporters normalized to that of R-Luc mRNA and set at 100 in the absence of the miRNAs. (C and G) Relative derepression of F-Luc activity for each condition. (D and H) Relative F-Luc mRNA levels. Throughout this study, error bars represent standard deviations from at least three independent experiments. Upper and lower dashed lines indicate maximal derepression and repression, respectively, observed in depleted cells. (I) A western blot showing that GW182 mutants were expressed at levels equivalent to that of the wild-type protein.