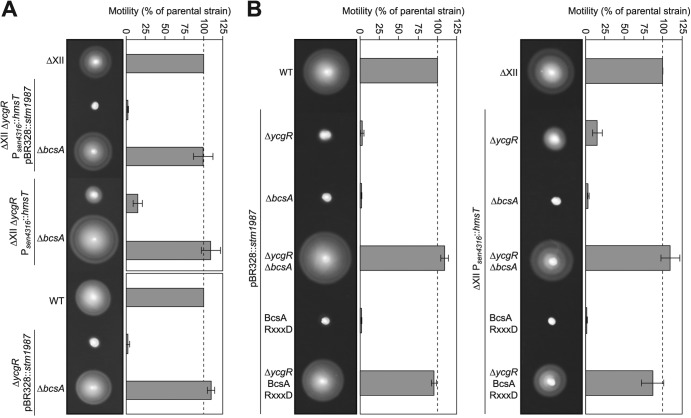
Fig 4.
C-di-GMP binding to BcsA is responsible for motility inhibition in the absence of YcgR. Representative swimming motility plates and quantitative measurement of motility after incubation at 23°C for 16 h are shown. (A) Swimming motility of a strain that expresses two unique sources of c-di-GMP and presents a mutation in ycgR was completely rescued by means of an additional mutation of the cellulose synthase-encoding gene, bcsA. The same result was obtained when the bcsA mutation was transduced to a ycgR single mutant overexpressing stm1987 and to ΔXII strain containing a ycgR mutation and overexpressing hmsT. (B) Deletion of both ycgR and bcsA in strains that present high levels of c-di-GMP is needed to recuperate swimming behavior. Restoration of motility is also achieved by mutating the c-di-GMP binding motif of the PilZ domain of BcsA.