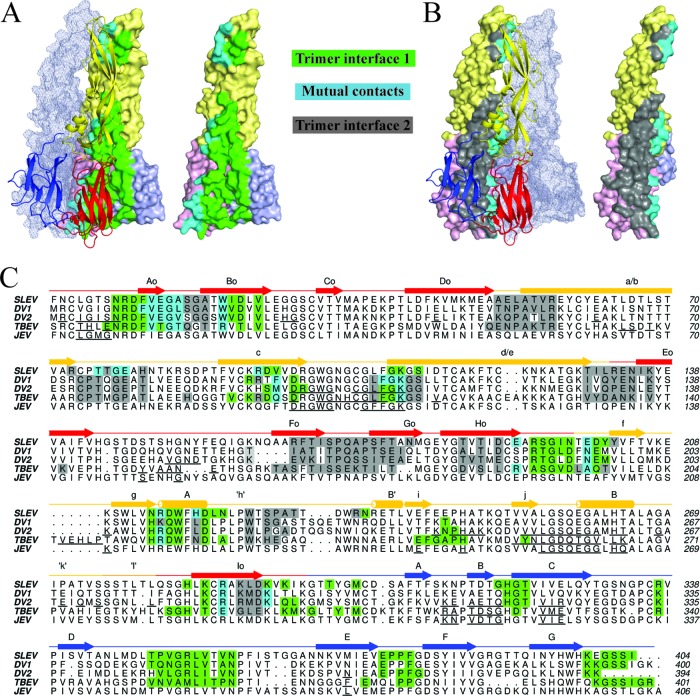
Fig 3.
Conservation of flavivirus E trimer interfaces. (A) The left panel displays trimer interface 1, formed by two E protomers. Residues contacted by the cartoon representation are highlighted on the surface-rendered protein. Green residues represent contacts exclusive to the surface-rendered protein, while cyan residues are contacts in both molecules at the interface. The third E protomer that does not make contacts at the highlighted interface is shown as a transparent mesh envelope. (B) The right panel displays trimer interface 2. Contacts in both molecules in this panel are also cyan, while residues exclusive to the surface-rendered protein are shown in gray. The protomer that does not make contacts at this interface is also shown in mesh representation. (C) A ClustalW sequence alignment of SLEV, DENV1, DENV2, and TBEV E. SLEV E domains I, II, and III are shown in red, yellow, and blue, respectively. The SLEV E secondary structure is displayed above the sequence with a straight line for coils/loops, arrows for strands, and cylinders for helices. Contact residues are highlighted in the same color scheme described for panels A and B. For JEV, DENV2, and TBEV E proteins with dimeric structures available, the dimer contacts are underlined in black.