Fig 1.
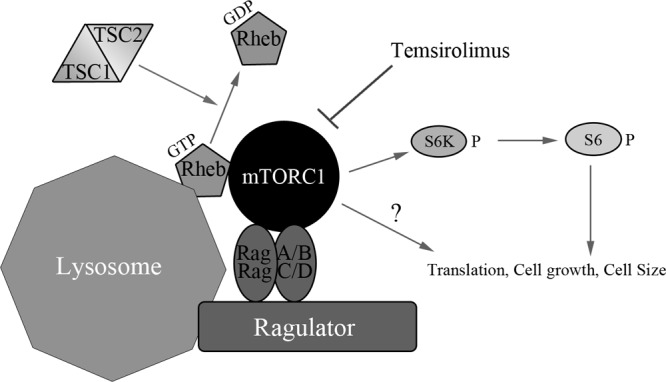
Outline of the general mTOR signaling pathway illustrating upstream mTOR activators and downstream mTOR substrates. The canonical signaling pathway senses growth factor stimulation at the plasma membrane, leading to the activation of phosphatidylinositol kinase and Akt and inhibition of the TSC1-TSC2 complex. Inhibition of the TSC1-TSC2 complex relieves its GTPase-activating protein (GAP) function, increasing the amount of GTP-bound Rheb in the cell. GTP-bound Rheb, the RagA/B-RagC/D heterodimer, and the ragulator complex(composed of LAMTOR1 [p18, c11orf59], LAMTOR2 [p14, ROBLD3], and LAMTOR3 [MP1, MAPKSP1]) help recruit and activate mTORC1 to the lysosomal membrane. Upon activation, mTORC1 phosphorylates and activates the p70/85 S6 kinases (S6K). Temsirolimus allosterically inhibits mTORC1 activity and reduces mTORC1 substrate phosphorylation. Active S6K phosphorylates ribosomal protein S6, promoting cap-dependent translation. In addition to S6K, mTORC1 also phosphorylates multiple substrates that regulate translation, cell growth, and cell size.
