Abstract
Transmission of drug-resistant HIV has been postulated to be a threat to current first-line antiretroviral therapy (ART) regimens and the efficacy of several antiretroviral-based preexposure prophylaxis (PrEP) strategies being tested. Here we evaluated the effect of the common tenofovir (TFV) resistance mutation K65R on vaginal HIV transmission. Our results demonstrate that despite no overt loss of overall replication competence in vivo, this mutation results in significantly reduced mucosal transmission. When transmitted, the mutant virus eventually reverted to the wild type in 2 of 3 animals examined.
TEXT
In the absence of a cure or vaccine, and despite valuable efforts toward better human immunodeficiency virus (HIV) education, including safe sex practices, the HIV epidemic continues to grow at a faster pace than the current availability of antiretroviral therapy (ART). For every two people who begin ART, five are newly infected (1). Of the people infected, only 47% have access to ART in low- and middle-income countries (2). There is a great need to prevent transmission of HIV. To address this need, extensive efforts are being made to develop and implement effective preexposure prophylaxis (PrEP) approaches. So far, the greatest progress has been made using antiretroviral drug-based treatment as prevention and PrEP (3, 4). When the patient has a positive diagnosis and access to a full ART regime under a doctor's guidance, early treatment is exceedingly effective for preventing transmission of HIV to uninfected partners (4). Unfortunately, a significant number of HIV-positive individuals do not know their infection status, especially during acute infection when transmission potential is highest, increasing the need for alternatives such as PrEP. Most current PrEP clinical trials are investigating the use of antiretroviral drugs either singularly or as a two-drug combination for systemic or topical use (3, 5–8). This raises an important concern with the dual use of antiretroviral drugs for both treatment and prevention: the consequences of the development and transmission of drug-resistant HIV.
HIV-1 develops resistance to virtually all drugs currently available for treatment (9, 10). For this reason, current ART therapies consist of a cocktail of multiple drugs with different classes of action to prevent or at least postpone the development of drug-resistant HIV within the patient's life span. Drug-resistant viruses can be transmitted (11–14). During new infections, certain mutations like M184V are rarely detected by routine genotyping, but significantly higher proportions can be detected using more-specific methodology (11, 13, 14). The inherent ability of replicating HIV to revert to a drug-sensitive genotype in the absence of drug pressure makes it difficult to study in patients, especially if (i) the time, duration, and route of infection are unknown, (ii) there is no way to prove ART-naïve status, and (iii) the HIV sequence in the infecting partner is unknown. Despite these difficulties, genotypic analysis of ART-naïve patients has provided evidence that drug-resistant HIV-1 is being transmitted and can result in treatment failure (15–21). Given that animal studies are the best option to overcome the inherent limitations of human studies (22), we utilized humanized mice to investigate in vivo transmission of drug-resistant HIV-1.
Tenofovir (TFV) is the drug most commonly used in clinical trials evaluating systemic and topical PrEP. Tenofovir disoproxil fumarate, the oral formulation of TFV, is also part of every DHHS-recommended first-line therapy (23). For this reason, we chose to study transmission of tenofovir-resistant HIV. The mutation of the lysine at amino acid position 65 in HIV reverse transcriptase to an arginine (K65R) confers resistance to tenofovir as well as other nucleoside reverse transcriptase inhibitors (NRTIs). For this reason, K65R is on both the WHO and International AIDS Society (IAS) surveillance lists for HIV genotyping (9, 10). There is clinical evidence that HIV containing the K65R mutation can be transmitted after mucosal exposure, albeit at a lower frequency than other mutations, like M184V (11, 13, 15, 20). To evaluate the role of this single amino acid mutation on mucosal HIV transmission, we introduced the K65R mutation (AAA to AGA) into a proviral clone of HIV-1JR-CSF (24). In addition, to differentiate the mutant virus from the parental clone after reversion, a second, silent mutation (TAT to TAC; tyrosine) was included to act as a molecular marker.
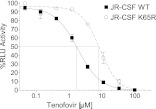
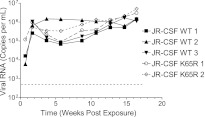
To confirm a decrease in the susceptibility of the mutant virus to TFV, we determined the in vitro 50% inhibitory concentration (IC50) for wild-type HIVJR-CSF and the isogenic mutant HIVJR-CSF K65R. The K65R mutation conferred a 4.7-fold increase in the in vitro IC50 for TFV, a change which is comparable to the 2- to 4-fold reduction in susceptibility reported previously (25, 26) (Fig. 1). Previous in vitro studies have shown that the K65R mutation reduces the function of viral reverse transcriptase (26, 27). It is unknown to what extent this defect affects viral replication in vivo. To test the in vivo replication capacity of HIVJR-CSF K65R, humanized mice (28, 29) were inoculated via intraperitoneal (i.p.) injection of 3 × 104 tissue culture infectious units (TCIU), and viral load in plasma was monitored over time (30). Longitudinal analysis of plasma viral load showed no difference in the in vivo replication of the K65R mutant and wild-type strains (Fig. 2) in this group of five animals, suggesting that there are not large differences in the in vivo fitness of the mutant virus. Sequence analysis of plasma virus RNA from HIV-1JR-CSF K65R-infected mice confirmed the presence of the K65R mutation 2 weeks postinfection. However, subsequent time points showed a population of wild-type virus. Sequence analysis indicated that reversion of the K65R mutation was always to the original sequence. It should be noted that the molecular marker, present only in the mutant virus, served to exclude the possibility of contamination with wild-type virus.
Fig 1.
Introduction of the K65R mutation into HIVJR-CSF results in a 4.7-fold increase in in vitro IC50 using a luciferase-based assay in TZM-bl indicator cells. Serial dilutions of tenofovir were applied to indicator cells in triplicate and allowed to incubate for 30 min before an equal number of tissue culture infectious units (TCIU) of either wild-type or mutant virus was applied to all wells. Two days later, the medium was removed, ONE-Glo reagent (Promega) was added, and the amount of luciferase activity was measured. Each curve was normalized to wells infected with that specific virus (wild-type or K65R virus) in the absence of the drug. RLU, relative light units.
Fig 2.
In vivo replication of HIVJR-CSF and HIVJR-CSF K65R after i.p. injection into humanized mice shows no overt difference in replication capacity. Humanized NOD/SCID/γ−/− mice (28, 29) were infected with equal amounts of either HIV-1JR-CSF or HIV-1JR-CSF K65R (3 × 104 TCIU) by i.p. injection. The course of infection was monitored by determining plasma viral loads. Dotted line indicates the limit of detection of the assay.
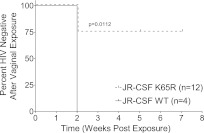
Having demonstrated the replication capacity of the K65R mutant virus in vivo, we next evaluated its capacity to transmit mucosally. For this purpose, we utilized BLT humanized mice (30). The female reproductive tract of BLT mice is reconstituted with all the cells relevant for HIV transmission, including human T cells, monocyte/macrophages, and dendritic cells (30, 31). BLT mice were vaginally exposed once to equal infectious doses of wild-type HIV-1JR-CSF or the isogenic K65R mutant virus (3.5 × 105 TCIU). Three independent exposures (n = 4) were performed on three different dates. The results of these vaginal exposures showed a dramatic decrease in the transmission efficiency of the K65R mutant virus (Fig. 3). Specifically, whereas all the mice exposed to the wild-type virus were infected (4/4), only 25% of the mice exposed to the mutant virus were infected (3/12). This difference in vaginal HIV transmission was highly statistically significant by log rank analysis (P = 0.011; Mantel-Cox). These results demonstrate that the K65R mutant is vaginally transmitted at a greatly reduced rate compared to that of the wild-type virus. Interestingly, these results seem at odds with those recently published by Cong et al. (22) using simian immunodeficiency virus (SIV). However, these results may be due to the facts that a different mutation was used and that additional fitness compensatory mutations were introduced into the provirus used by Cong et al. (22).
Fig 3.
The K65R mutation reduces vaginal transmission efficiency of HIV-1 by 75%. Humanized BLT mice were prepared and validated as previously described (30, 31, 38). Mice were exposed vaginally to a single dose of HIV-1JR-CSF or HIV-1JR-CSF K65R (3.5 × 105 TCIU). Infection was monitored as a function of viral load in plasma. The Kaplan-Meyer plot shows the percentage of HIV-negative mice as a function of the number of weeks postexposure until the first peripheral blood HIV-1 detection. In 3/12 (25%) mice, viral load was readily detectable 2 weeks postexposure. In 9/12 (75%) mice exposed to the K65R mutant, no viral load was detected at any time point analyzed and no viral DNA was found in tissues at harvest, confirming the lack of transmission.
To determine if the transmitted virus contained the K65R mutation, plasma viral RNA was sequenced at different times after exposure. Four weeks postexposure, we noted the presence of only mutant virus in one mouse (M1), the presence of only wild-type (reverted) virus in a second mouse (M2), and the presence of both mutant virus and wild-type (reverted) virus populations in a third mouse (M3). Longitudinal analysis of the virus found in the plasma of one of the infected mice (M3) showed the presence of both mutant and wild-type viruses at weeks 4 and 6 postinfection and the presence of wild-type virus at all subsequent time points (Table 1). Cervicovaginal lavage (CVL) fluid from this mouse also showed the presence of both wild-type and mutant virus 4 weeks postinfection. Subsequently, only the wild-type virus was found in the CVL fluid (Table 1). Analysis of the virus present in the different tissues from two of the infected mice generally reflected what was observed in the periphery. However, in one mouse, the mutant virus was found in the plasma but all tissues analyzed contained both the wild-type and mutant viruses. Interestingly, analysis of the virus present in tissues 14 weeks postinfection showed the wild-type virus in all tissues except the thymic organoid, in which both drug-resistant and wild-type viruses were found (Table 1). These results are consistent with the hypothesis of Weinberg et al. suggesting that transmitted viruses that contain reversible mutations become archived in lymphocyte reservoirs (14).
Table 1.
Sequence analysis demonstrates reversion of the K65R mutation over time in peripheral blood, cervicovaginal lavage fluid, and tissues of infected BLT micea
| Mouse | Wk postexposure | Amino acid(s) at position 65 in sample from: |
|||||
|---|---|---|---|---|---|---|---|
| Peripheral blood | Vaginal lavage fluid | FRT | Lymph node | Organoid implant | Lung | ||
| M1 | 4 | R only | NA | NA | K and R | K and R | K and R |
| M2 | 4 | K only | NA | NA | K only | K only | K only |
| M3 | 4 | K and R | K and R | ||||
| 6 | K and R | K only | |||||
| 9 | K only | K only | |||||
| 13 | K only | K only | |||||
| 14 | K only | K only | K only | K only | K and R | K only | |
Bone marrow/liver/thymus mice were exposed once intravaginally to the mutant virus. Infection was monitored in plasma by determining the viral load. Two mice were harvested 4 weeks postinfection (M1 and M2), and one was harvested 14 weeks postexposure (M3). Peripheral blood and vaginal lavage fluid samples from this mouse were collected longitudinally. FRT, female reproductive tract; K, lysine; R, arginine; NA, not available. PCR primer sets used to amplify the reverse transcriptase (RT) are as follows: outer/first reaction, 5′-GCTCTATTAGATACAGGAGC-3′ and 5′-CCTAATGCATATTGTGAGTCTG-3′; inner/second reaction, 5′-GTAGGACCTACACCTGTCAAC-3′ and 5′-CCTGCAAAGCTAGGTGAATTGC-3′. Amplification products were sequenced in bulk.
In summary, the topical or systemic use of antiretroviral drugs for the purpose of preventing HIV acquisition has the potential to curtail the spread of AIDS, and some PrEP strategies have shown great promise (4, 5, 7, 32, 33). The fact that tenofovir is a successful first-line drug for the treatment of HIV infection has made this compound the drug of choice for most prevention trials (34). However, this dual-use approach is not without risk, as there is significant potential to expand the pool of drug resistance in communities utilizing PrEP (32, 35). Here we tested K65R-mutated HIV-1 in humanized mice and found that, as in humans, the HIV carrying the K65R mutation (i) is replication competent (Fig. 2), (ii) is present in cervicovaginal secretions (Table 1), and (iii) reverts to the wild type in the absence of drug selection although the mutant virus remains detectable (Table 1). Finally, we tested the ability of K65R mutant HIV to transmit vaginally and found that it can transmit, albeit at a significantly lower efficiency than that for the wild type (Fig. 3). At this point, the molecular basis for this lower transmission is not known. However, analysis of the K65R mutant has shown that it has a decreased replication capacity compared to the wild type in several in vitro model systems (36, 37). Overall, our results demonstrate that if this tenofovir-resistant virus is present in the transmitting partner, there is the potential for the mutant virus to be transmitted to the uninfected partner with lower efficiency than wild-type HIV-1.
ACKNOWLEDGMENTS
This work was supported in part by the U19 AI082637 Combination HIV Antiretroviral Rectal Microbicide (CHARM) Program (to J.V.G.), the University of North Carolina Center for AIDS Research (P30AI50410), grant AI73146 (to J.V.G.), and the National Institutes of Health training grant T32CA9156-37 and F32AI100775 (to M.D.S.).
We thank Ian McGowan, Peter Anton, and Charlene Dezzutti for reading the manuscript and providing valuable suggestions. We thank I. Chen for providing the JR-CSF plasmid via the AIDS Research and Reagent Program. We thank former and current lab members and veterinary technicians at the UNC Division of Laboratory Animal Medicine for their assistance with various technical aspects of this work.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. UNAIDS 2009. AIDS epidemic update: December 2009. UNAIDS, World Health Organization, Geneva, Switzerland: http://data.unaids.org/pub/report/2009/jc1700_epi_update_2009_en.pdf [Google Scholar]
- 2. UNAIDS 2011. UNAIDS World AIDS Day report. UNAIDS, World Health Organization, Geneva, Switzerland: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/jc2216_worldaidsday_report_2011_en.pdf [Google Scholar]
- 3. Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, CAPRISA 004 Trial Group 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, HPTN 052 Study Team 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 367:423–434 [DOI] [PubMed] [Google Scholar]
- 7. Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. 2012. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS 26:F13–F19 [DOI] [PubMed] [Google Scholar]
- 9. Bennett DE, Camacho RJ, Otelea D, Kuritzkes DR, Fleury H, Kiuchi M, Heneine W, Kantor R, Jordan MR, Schapiro JM, Vandamme AM, Sandstrom P, Boucher CA, van DE Vijver D, Rhee SY, Liu TF, Pillay D, Shafer RW. 2009. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 4:e4724 doi:10.1371/journal.pone.0004724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson VA, Calvez V, Gunthard HF, Paredes R, Pillay D, Shafer R, Wensing AM, Richman DD. 2011. 2011 update of the drug resistance mutations in HIV-1. Top. Antivir. Med. 19:156–164 [PMC free article] [PubMed] [Google Scholar]
- 11. Brenner BG, Roger M, Moisi DD, Oliveira M, Hardy I, Turgel R, Charest H, Routy J-P, Wainberg MA, Montreal PHI Cohort and HIV Prevention Study Groups 2008. Transmission networks of drug resistance acquired in primary/early stage HIV infection. AIDS 22:2509–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hightow-Weidman LB, Hurt CB, Phillips G, II, Jones K, Magnus M, Giordano TP, Outlaw A, Ramos D, Enriquez-Bruce E, Cobbs W, Wohl A, Tinsle M. 2011. Transmitted HIV-1 drug resistance among young men of color who have sex with men: a multicenter cohort analysis. J. Adolesc. Health 48:94–99 [DOI] [PubMed] [Google Scholar]
- 13. Metzner KJ, Bonhoeffer S, Fischer M, Karanicolas R, Allers K, Joos B, Weber R, Hirschel B, Kostrikis LG, Gunthard HF, Swiss HIV Cohort Study 2003. Emergence of minor populations of human immunodeficiency virus type 1 carrying the M184V and L90M mutations in subjects undergoing structured treatment interruptions. J. Infect. Dis. 188:1433–1443 [DOI] [PubMed] [Google Scholar]
- 14. Wainberg MA, Moisi D, Oliveira M, Toni TD, Brenner BG. 2011. Transmission dynamics of the M184V drug resistance mutation in primary HIV infection. J. Antimicrob. Chemother. 66:2346–2349 [DOI] [PubMed] [Google Scholar]
- 15. Bansal V, Metzner KJ, Niederost B, Leemann C, Boni J, Gunthard HF, Fehr JS. 2011. Minority K65R variants and early failure of antiretroviral therapy in HIV-1-infected Eritrean immigrant. Emerg. Infect. Dis. 17:1966–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borroto-Esoda K, Waters JM, Bae AS, Harris JL, Hinkle JE, Quinn JB, Rousseau FS. 2007. Baseline genotype as a predictor of virological failure to emtricitabine or stavudine in combination with didanosine and efavirenz. AIDS Res. Hum. Retroviruses 23:988–995 [DOI] [PubMed] [Google Scholar]
- 17. Frentz D, Boucher CA, van de Vijver DA. 2012. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev. 14:17–27 [PubMed] [Google Scholar]
- 18. Huang HY, Daar ES, Sax PE, Young B, Cook P, Benson P, Cohen C, Scribner A, Hu H. 2008. The prevalence of transmitted antiretroviral drug resistance in treatment-naive patients and factors influencing first-line treatment regimen selection. HIV Med. 9:285–293 [DOI] [PubMed] [Google Scholar]
- 19. Kuritzkes DR, Lalama CM, Ribaudo HJ, Marcial M, Meyer WA, III, Shikuma C, Johnson VA, Fiscus SA, D'Aquila RT, Schackman BR, Acosta EP, Gulick RM. 2008. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J. Infect. Dis. 197:867–870 [DOI] [PubMed] [Google Scholar]
- 20. Li JF, Lipscomb JT, Wei X, Martinson NA, Morris L, Heneine W, Johnson JA. 2011. Detection of low-level K65R variants in nucleoside reverse transcriptase inhibitor-naive chronic and acute HIV-1 subtype C infections. J. Infect. Dis. 203:798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, Koup RA, Mellors JW, Connick E, Conway B, Kilby M, Wang L, Whitcomb JM, Hellmann NS, Richman DD. 2002. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 347:385–394 [DOI] [PubMed] [Google Scholar]
- 22. Cong ME, Youngpairoj AS, Aung W, Sharma S, Mitchell J, Dobard C, Heneine W, Garcia-Lerma JG. 2011. Generation and mucosal transmissibility of emtricitabine- and tenofovir-resistant SHIV162P3 mutants in macaques. Virology 412:435–440 [DOI] [PubMed] [Google Scholar]
- 23. Panel on Antiretroviral Guidelines for Adults and Adolescents 2012. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. US Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf [Google Scholar]
- 24. Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819–822 [DOI] [PubMed] [Google Scholar]
- 25. Gilead Sciences 2011. Truvada package insert. Gilead Sciences, Foster City, CA [Google Scholar]
- 26. Wainberg MA, Miller MD, Quan Y, Salomon H, Mulato AS, Lamy PD, Margot NA, Anton KE, Cherrington JM. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87–94 [DOI] [PubMed] [Google Scholar]
- 27. Wagner BG, Garcia-Lerma JG, Blower S. 2012. Factors limiting the transmission of HIV mutations conferring drug resistance: fitness costs and genetic bottlenecks. Sci. Rep. 2:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dash PK, Gorantla S, Gendelman HE, Knibbe J, Casale GP, Makarov E, Epstein AA, Gelbard HA, Boska MD, Poluektova LY. 2011. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J. Neurosci. 31:3148–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. 2009. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/−, and C.B-17-scid/bg immunodeficient mice. Hum. Immunol. 70:790–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, Zein S, Powell DA, Wahl A, Kwak YT, Welch BD, Kay MS, Payne DA, Gallay P, Appella E, Estes JD, Lu M, Garcia JV. 2011. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J. Virol. 85:7582–7593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olesen R, Wahl A, Denton PW, Garcia JV. 2011. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J. Reprod. Immunol. 88:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hurt CB, Eron JJ, Jr, Cohen MS. 2011. Pre-exposure prophylaxis and antiretroviral resistance: HIV prevention at a cost? Clin. Infect. Dis. 53:1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veronese F, Anton P, Fletcher CV, DeGruttola V, McGowan I, Becker S, Zwerski S, Burns D, Workshop Organizing Committee 2011. Implications of HIV PrEP trials results. AIDS Res. Hum. Retroviruses 27:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen MS, Muessig KE, Smith MK, Powers K, Kashuba AD. 2012. Antiviral agents and HIV prevention: controversies, conflicts and consensus. AIDS 26:1585–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen MS, Baden LR. 2012. Preexposure prophylaxis for HIV—where do we go from here? N. Engl. J. Med. 367:459–461 [DOI] [PubMed] [Google Scholar]
- 36. Chunduri H, Crumpacker C, Sharma PL. 2011. Reverse transcriptase mutation K65N confers a decreased replication capacity to HIV-1 in comparison to K65R due to a decreased RT processivity. Virology 414:34–41 [DOI] [PubMed] [Google Scholar]
- 37. Perez-Bercoff D, Wurtzer S, Compain S, Benech H, Clavel F. 2007. Human immunodeficiency virus type 1: resistance to nucleoside analogues and replicative capacity in primary human macrophages. J. Virol. 81:4540–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12:1316–1322 [DOI] [PubMed] [Google Scholar]